
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 69P
How many signals would the product of the following reaction show in
- a. its 1H NMR spectrum?
- b. its 13C NMR spectrum?
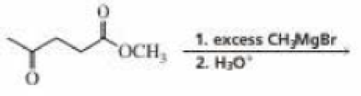
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the following two compounds, indicate and label where the electrophilic and nucleophilic
sites are.
요
N
Please correct answer and don't use Hand rating
None
Chapter 16 Solutions
EBK ORGANIC CHEMISTRY
Ch. 16.1 - Give two names for each of the following:Ch. 16.1 - Prob. 2PCh. 16.1 - Name the following:Ch. 16.2 - Prob. 4PCh. 16.4 - What products are formed when the following...Ch. 16.4 - We saw on the previous page that...Ch. 16.4 - a. How many stereoisomers are obtained from the...Ch. 16.4 - Prob. 9PCh. 16.4 - Write the mechanism for the reaction of acetyl...Ch. 16.4 - Prob. 11P
Ch. 16.4 - Show how the following compounds can be...Ch. 16.4 - Prob. 13PCh. 16.4 - Prob. 14PCh. 16.4 - In the mechanism for cyanohydrin formation, why is...Ch. 16.4 - Prob. 16PCh. 16.4 - Prob. 17PCh. 16.4 - Show two ways to convert an alkyl halide into a...Ch. 16.5 - Prob. 20PCh. 16.5 - Prob. 21PCh. 16.5 - Prob. 22PCh. 16.5 - Prob. 23PCh. 16.6 - Prob. 24PCh. 16.7 - What reducing agents should be used to obtain the...Ch. 16.7 - Prob. 26PCh. 16.8 - Prob. 27PCh. 16.8 - Prob. 28PCh. 16.8 - Prob. 29PCh. 16.8 - The pKa of protonated acetone is about 7.5. and...Ch. 16.8 - Prob. 31PCh. 16.8 - Prob. 32PCh. 16.8 - Prob. 33PCh. 16.8 - Excess ammonia must be used when a primary amine...Ch. 16.8 - The compounds commonly known as amino acids are...Ch. 16.9 - Hydration of an aldehyde is also catalyzed by...Ch. 16.9 - Which ketone forms the most hydrate in an aqueous...Ch. 16.9 - When trichloroacetaldehyde is dissolved in water,...Ch. 16.9 - Which of the following are a. hermiacetals? b....Ch. 16.9 - Prob. 40PCh. 16.9 - Explain why an acetal can be isolated but most...Ch. 16.10 - Prob. 42PCh. 16.10 - Prob. 43PCh. 16.10 - What products would be formed from the proceedings...Ch. 16.10 - a. In a six-step synthesis, what is the yield of...Ch. 16.10 - Show how each of the following compounds could be...Ch. 16.12 - Prob. 47PCh. 16.13 - Prob. 49PCh. 16.14 - Prob. 50PCh. 16.15 - Prob. 51PCh. 16.16 - Prob. 52PCh. 16 - Draw the structure for each of the following: a....Ch. 16 - Prob. 54PCh. 16 - Prob. 55PCh. 16 - Prob. 56PCh. 16 - a. Show the reagents required to form the primary...Ch. 16 - Prob. 58PCh. 16 - Prob. 59PCh. 16 - Using cyclohexanone as the starting material,...Ch. 16 - Propose a mechanism for each of the following...Ch. 16 - Show how each of the following compounds can be...Ch. 16 - Fill in the boxes:Ch. 16 - Prob. 64PCh. 16 - Identify A through O:Ch. 16 - Prob. 66PCh. 16 - Prob. 67PCh. 16 - Prob. 68PCh. 16 - How many signals would the product of the...Ch. 16 - Prob. 70PCh. 16 - Prob. 71PCh. 16 - Prob. 72PCh. 16 - Prob. 73PCh. 16 - Prob. 74PCh. 16 - Prob. 75PCh. 16 - Prob. 76PCh. 16 - Prob. 77PCh. 16 - Prob. 78PCh. 16 - Draw structure for A-D for each of the following:Ch. 16 - Prob. 80PCh. 16 - a. Propose a mechanism for the following reaction:...Ch. 16 - Prob. 82PCh. 16 - A compound gives the following IR spectrum. Upon...Ch. 16 - How can be following compounds be prepared from...Ch. 16 - Prob. 85PCh. 16 - Prob. 86PCh. 16 - Prob. 87PCh. 16 - In the presence of an acid catalyst, acetaldehyde...Ch. 16 - Prob. 89PCh. 16 - Prob. 90PCh. 16 - Prob. 91PCh. 16 - A compound reacts with methylmagnesium bromide...Ch. 16 - Show how each of the following compounds can be...Ch. 16 - Prob. 94PCh. 16 - The pKa values of the carboxylic acid groups of...Ch. 16 - The Baylis-Hillman reaction is a DABCO...Ch. 16 - Prob. 97PCh. 16 - Prob. 98P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forward3. Propose a synthesis for the following transformation. Do not draw an arrow-pushing mechanism below, but make sure to draw the product of each proposed step (3 points). CN + En CNarrow_forward3) Propagation of uncertainty. Every measurement has uncertainty. In this problem, we'll evaluate the uncertainty in every step of a titration of potassium hydrogen phthalate (a common acid used in titrations, abbreviated KHP, formula CsH5KO4) with NaOH of an unknown concentration. The calculation that ultimately needs to be carried out is: concentration NaOH 1000 x mass KHP × purity KHP molar mass KHP x volume NaOH Measurements: a) You use a balance to weigh 0.3992 g of KHP. The uncertainty is ±0.15 mg (0.00015 g). b) You use a buret to slowly add NaOH to the KHP until it reaches the endpoint. It takes 18.73 mL of NaOH. The uncertainty of the burst is 0.03 mL.. c) The manufacturer states the purity of KHP is 100%±0.05%. d) Even though we don't think much about them, molar masses have uncertainty as well. The uncertainty comes from the distribution of isotopes, rather than random measurement error. The uncertainty in the elements composing KHP are: a. Carbon: b. Hydrogen: ±0.0008…arrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardHow would you use infrared spectroscopy to distinguish between the following pairs of constitutional isomers? (a) CH3C=CCH3 || and CH3CH2C=CH (b) CH3CCH=CHCH3 and CH3CCH2CH=CH2 Problem 12-41 The mass spectrum (a) and the infrared spectrum (b) of an unknown hydrocarbon are shown. Propose as many structures as you can. (a) 100 Relative abundance (%) 80 60 60 40 200 20 (b) 100 Transmittance (%) 10 20 20 80- 60- 40- 20 40 60 80 100 120 140 m/z 500 4000 3500 3000 2500 2000 1500 Wavenumber (cm-1) 1000arrow_forwardPropagation of uncertainty. You have a stock solution certified by the manufacturer to contain 150.0±0.03 µg SO42-/mL. You would like to dilute it by a factor of 100 to obtain 1.500 µg/mL. Calculate the uncertainty in the two methods of dilution below. Use the following uncertainty values for glassware: Glassware Uncertainty (assume glassware has been calibrated and treat the values below as random error) 1.00 mL volumetric pipet 0.01 mL 10.00 mL volumetric pipet 0.02 mL 100.00 mL volumetric flask 0.08 mL Transfer 10.00 mL with a volumetric pipet and dilute it to 100 mL with a volumetric flask. Then take 10.00 mL of the resulting solution and dilute it a second time with a 100 mL flask. 2. Transfer 1.00 mL with a volumetric pipet and dilute it to 100 mL with a volumetric flask.arrow_forward
- Draw all resonance structures for the following ion: CH₂ Draw all resonance structures on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars, including charges where needed. The single bond is active by default. 2D ד CONT HD EXP CON ? 1 [1] Α 12 Marvin JS by Chemaxon A DOO H C N Br I UZ OSPFarrow_forwardWhat is the average mass of the 10 pennies? Report your value with correct significant figures. What is the error (uncertainty) associated with each mass measurement due to the equipment? What is the uncertainty associated with the average value? Note that the uncertainty of the balance will propagate throughout the calculation. What is the standard deviation of the 10 mass measurements? Explain the difference between the propagated uncertainty and the standard deviation. Which number would you use to describe the uncertainty in the measurement? Calculate the total mass of the pennies with associated uncertainty. Calculate the average density of a penny based on these data. Propagate the uncertainty values for both mass and volume in your calculations.arrow_forwardCan you help me and explain the answers please.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY