
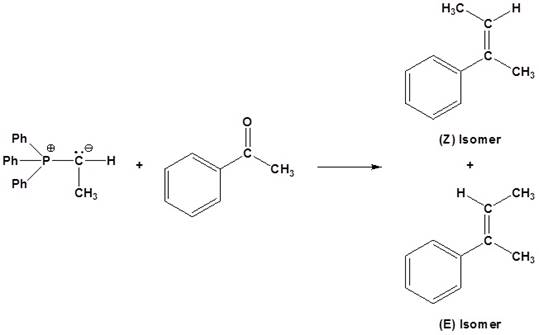
(a)
Interpretation:
The set of reagents satisfying the given conditions used for the synthesis of the given
Concept introduction:
An
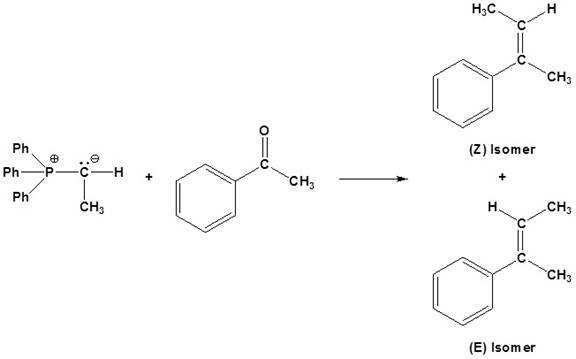
(b)
Interpretation:
The
Concept introduction:
An aldehyde or a ketone reacts with phosphonium ylide to form an alkene. This reaction is known as wittig reaction.
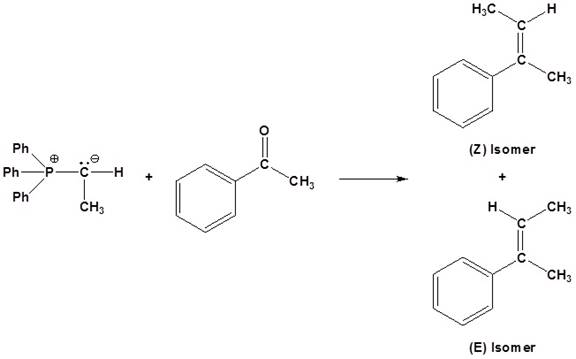
(c)
Interpretation:
The reagents used for the synthesis of given set of alkenes should be determined.
Concept introduction:
An aldehyde or a ketone reacts with phosphonium ylide to form an alkene. This reaction is known as wittig reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
EBK ORGANIC CHEMISTRY
- Predict the major organic product(s) of the following reactions. Include stereochemistry when necessary. Write NR if no reaction, try to explain.arrow_forwardQ2: Explain why epoxides that react in an SN1 manner will not show any stereochemical inversion in the product. Q3: Rationalize why Alcohol B will react under the indicated reaction conditions, but Alcohol A will not. A ☑ OH B OH PBr3 R-Brarrow_forwardQ1: Predict the major organic product(s) of the following reactions. Include stereochemistry when necessary. Write NR if no reaction, try to explain. 1.) LDA, THF 2.) СОН CI OH H2SO4, heat OH m...... OH 1.) PCC, CH2Cl2 2.) CH3CH2MgBr, THF 3.) H3O+ 4.) TsCl, pyr 5.) tBuOK, tBuOH 1.) SOCI 2, CHCI 3 2.) CH3CH2ONA, DMF OH 1.) HBr 2.) Mg, THF 3.) H₂CO, THE 4.) H3O+ OH NaH, THFarrow_forward
- Problem 6-29 Identify the functional groups in the following molecules, and show the polarity of each: (a) CH3CH2C=N CH, CH, COCH (c) CH3CCH2COCH3 NH2 (e) OCH3 (b) (d) O Problem 6-30 Identify the following reactions as additions, eliminations, substitutions, or rearrangements: (a) CH3CH2Br + NaCN CH3CH2CN ( + NaBr) Acid -OH (+ H2O) catalyst (b) + (c) Heat NO2 Light + 02N-NO2 (+ HNO2) (d)arrow_forwardPredict the organic product of Y that is formed in the reaction below, and draw the skeletal ("line") structures of the missing organic product. Please include all steps & drawings & explanations.arrow_forwardPlease choose the best reagents to complete the following reactionarrow_forward
- Problem 6-17 Look at the following energy diagram: Energy Reaction progress (a) Is AG for the reaction positive or negative? Label it on the diagram. (b) How many steps are involved in the reaction? (c) How many transition states are there? Label them on the diagram. Problem 6-19 What is the difference between a transition state and an intermediate? Problem 6-21 Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall AG°, transition states, and intermediate. Is AG° positive or negative? Problem 6-23 Draw an energy diagram for a reaction with Keq = 1. What is the value of AG° in this reaction?arrow_forwardProblem 6-37 Draw the different monochlorinated constitutional isomers you would obtain by the radical chlorination of the following compounds. (b) (c) Problem 6-39 Show the structure of the carbocation that would result when each of the following alkenes reacts with an acid, H+. (a) (b) (c)arrow_forwardPlease draw the major product of this reaction. Ignore inorganic byproducts and the carboxylic side productarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

