Problem 1PS: Write the formula and the give the name of the conjugate base of each of the following acids. a) HCN... Problem 2PS: Write the formula and give the name of the conjugate base of each of the following acids. a) NH3 b)... Problem 3PS: What are the products of each of the following acid-base reactions? Indicate the acid and its... Problem 4PS: What are the products of each of the following acid-base reactions? Indicate the acid and its... Problem 5PS: Write balanced equations showing how the hydrogen oxalate ion, HC2O4, can be both a Bronsted acid... Problem 6PS: Write a balanced equation showing how the HPO42 ion of sodium hydrogen phosphate Na2HPO4 can be a... Problem 7PS: In each of the following acid-base reactions, identify the Brnsted acid and base on the left and... Problem 8PS: In each of the following acid-base reactions, identify the Brnsted acid and base on the left and... Problem 9PS: An aqueous solution has a pH of 3.75. What is the hydronium ion concentration of the solution? Is it... Problem 10PS: A saturated solution of milk of magnesia. Mg(OH)2, has a pH of 10.52. What is the hydronium ion... Problem 11PS: What is the pH of a 0.0075 M solution of HCl? What is the hydroxide ion concentration of the... Problem 12PS: What is the pH of a 1.2 104 M solution of KOH? What is the hydronium ion concentration of the... Problem 13PS: What is the pH of a 0.0015 M solution of Ba(OH)2? Problem 14PS: The pH of a solution of Ba(OH)2 is 10.66 at 25 . What is the hydroxide ion concentration in the... Problem 15PS: Several acids are listed here with their respective equilibrium constants: C6H5OH(aq) + H2O() ... Problem 16PS: Several acids are listed here with their respective equilibrium constants: HF(aq) + H2O() H3O+(aq)... Problem 17PS: Which of the following ions or compounds has the strongest conjugate base? Briefly explain your... Problem 18PS: Which of the following compounds or ions has the weakest conjugate base? Briefly explain your... Problem 19PS: Which of the following compounds or ions has the weakest conjugate acid? Briefly explain your... Problem 20PS: Which of the following compounds or ion has the strongest conjugate acid? Briefly explain your... Problem 21PS: Dissolving K2CO3 in water gives a basic solution. Rite a balanced equation showing how this can... Problem 22PS: Dissolving ammonium bromide in water gives an acidic solution. Write a balanced equation showing... Problem 23PS: If each of the salts listed here were dissolved in water to give 0.10M solution, which solution... Problem 24PS: Which of the following common food additives gives a basic solution when dissolved in water? (a)... Problem 25PS Problem 26PS Problem 27PS Problem 28PS: An organic acid has pKa = 8.95. What is its Ka value? Where does the acid fit in Table 16.2? TABLE... Problem 29PS Problem 30PS: Which is the stronger of the following two acids? (a) acetic acid, CH3CO2H, Ka = 1.8 105 (b)... Problem 31PS: Chloroacetic acid (ClCH2CO2H) has Ka = 1.41 103. What is the value of Kb for the chloroacetate ion... Problem 32PS: A weak base has Kb = 1.5 109. What is the value of Ka for the conjugate acid? Problem 33PS: The trimethylammonium ion, (CH3)3NH+, is the conjugate acid of the weak base trimethylamine,... Problem 34PS: The chromium(III) ion in water, [Cr(H2O)6]3+. Is a weak acid with pKa = 3.95. What is the value of... Problem 35PS: Acetic acid and sodium hydrogen carbonate, NaHCO3, are mixed in water. Write a balanced equation for... Problem 36PS: Ammonium chloride and sodium dihydrogen phosphate, NaH2PO4, are mixed in water. Write a balanced... Problem 37PS: For each of the following reactions, predict whether the equilibrium lies predominantly to the left... Problem 38PS: For each of the following reactions, predict whether the equilibrium lies predominantly to the left... Problem 39PS: Equal molar quantities of sodium hydroxide and sodium hydrogen phosphate (Na2HPO4) are mixed. (a)... Problem 40PS: Equal molar quantities of hydrochloric acid and sodium hypochlorite (NaClO) are mixed. (a) Write the... Problem 41PS: Equal molar quantities of acetic acid and sodium hydrogen phosphate (Na2HPO4) are mixed. (a) Write a... Problem 42PS: Equal molar quantities of ammonia and sodium dihydrogen phosphate (NaH2PO4) are mixed. (a) Write a... Problem 43PS: A 0.015 M solution of hydrogen cyanate, HOCN, has a pH of 2.67. (a) What is the hydronium ion... Problem 44PS: A 0.10 M solution of chloroacetic acid, CICH2CO2H, has a pH of 1.95. Calculate Ka for the acid. Problem 45PS: A 0.025 M solution of hydroxyl amine has a pH of 9.11. What is the value of Kb for this weak base?... Problem 46PS: Methylamine, CH3NH2, is a weak base. CH3NH2(aq) + H2O() CH3NH3+(aq) + OH(aq) If the pH of a 0.065 M... Problem 47PS: A 2.5 103 M solution of an unknown acid has a pH of 3.80 at 25 C. (a) What is the hydronium ion... Problem 48PS: A 0.015M solution of a base has a pH of 10.09 a) What are the hydronium and hydroxide ion... Problem 49PS: What are the equilibrium concentrations of hydronium ion, acetate ion and acetic acid in a 0.20 M... Problem 50PS: The ionizations constant of a very weak acid, HA is 4.0109.Calculate the equilibrium concentrations... Problem 51PS: What are the equilibrium concentration of H3O+, CN and HCN in a 0.025 M solution of HCN? What is the... Problem 52PS: Phenol (C6H5OH) commonly called carbolic acid is a weak organic acid... Problem 53PS: What are the equilibrium concentrations of NH3,NH4+ and OH in a 0.15M solution of ammonia? What is... Problem 54PS: A hypothetical weak base has Kb=5.0104.Calculate the equilibrium concentrations of the base, its... Problem 55PS: The weak base methylamine, CH3NH2, has Kb=4.2104. It reacts with water according to the equation.... Problem 56PS: Calculate the pH of a 0.12 M aqueous solution of the base aniline, C6H5NH2(Kb=4.01010).... Problem 57PS: Calculate the pH of a 0.0010 M aqueous solution of HF. Problem 58PS: A solution of hydrofluoric acid, HF, has a pH of 2.30. Calculate the equilibrium concentration of... Problem 59PS: Calculate the hydronium ion concentration and pH in a 0.20 M solution of ammonium chloride NH4Cl. Problem 60PS: Calculate the hydronium ion concentration and pH for a 0.015M solution of sodium formate NaHCO3. Problem 61PS: Sodium cyanide is the salt of the weak acid HCN. Calculate the concentrations of H3O+,OH,HCN and Na+... Problem 62PS: The sodium salt of propionic acid, NaCH3CH2CO2 is used as an antifungal agent by veterinarians.... Problem 63PS: Calculate the hydronium ion concentration and pH of the solution that results when 22.0mL of 0.15M... Problem 64PS: Calculate the hydronium ion concentration and the pH when 50.0mL of 0.40M NH3 is mixed with 50.0mL... Problem 65PS: For each of the following cases, decide whether the pH is less than 7 equal to 7 or greater than 7.... Problem 66PS: For each of the following cases, decide whether the pH is less than 7, equal to 7 or greater than 7.... Problem 67PS: Oxalic acid, H2C2O4, is a diprotic acid. Write a chemical equilibrium expression for each ionization... Problem 68PS: Sodium carbonate is a diprotic base. Write a chemical equilibrium expression for each of the two... Problem 69PS: Prove that Ka1 Kb2 = Kw for oxalic acid H2C2O4, by adding the chemical equilibrium expressions that... Problem 70PS: Prove that Ka3 Kb1 = Kw for phosphoric acid, H3PO4, by adding the chemical equilibrium expressions... Problem 71PS: Sulphurous acid, H2SO3, is a weak acid capable of providing two H+ ions. (a) What is the pH of a... Problem 72PS: Ascorbic acid (vitamin C, C6H8O6) is a diprotic acid (Ka1 = 6.8 105 and Ka2 = 2.7 1012). What is... Problem 73PS: Hydrazine, N2H4, can interact with water in two steps. N2H4(aq) + H2O() N2H5+(aq) + OH(aq) Kb1 =... Problem 74PS: Ethylene diamine, H2NCH2CH2NH2, can interact with water in two steps, forming OH in each step... Problem 75PS: Which should be stronger acid, HOCN or HCN? Explain briefly. (In HOCN, the H+ ion is attached to the... Problem 76PS Problem 77PS: Explain why benzene sulfonic acid is a Brnsted acid. Problem 78PS: The structure of ethylene diamine is illustrated in study question 76. Is this compound a Brnsted... Problem 79PS: Decide whether each of the following substances should be classified as a Lewis acid or a Lewis... Problem 80PS: Decide whether each of the following substances should be classified as a Lewis acid or a Lewis... Problem 81PS: Carbon monoxide forms complexes with low-valent metals. For example, Ni(CO)4 and Fe(CO)5 are well... Problem 82PS: Trimethylamine, (CH3)3N, is a common reagent. It interacts readily with diborane gas, B2H6. The... Problem 83GQ: About this time, you may be wishing you had an aspirin. Aspirin is an organic acid with a Ka of 3.27... Problem 84GQ: Consider the following ions: NH4+, CO32, Br, S2, and ClO4. (a) Which of these ions in water gives an... Problem 85GQ: A 2.50 g sample of a solid that could be Ba(OH)2 or Sr(OH)2 was dissolved in enough water to make... Problem 86GQ: In a particular solution, acetic acid is 11% ionized at 25 C. Calculate the pH of the solution and... Problem 87GQ: Hydrogen, H2S, and sodium acetate, NaCH3CO2 are mixed in water. Using Table 16.2, write a balanced... Problem 88GQ: For each of the following reactions predict whether the equilibrium lies predominantly to the left... Problem 89GQ: A monoprotic acid HX has Ka = 1.3 103. Calculate the equilibrium concentration of HX and H3O+ and... Problem 90GQ: Arrange the following 0.10M solutions in order of increasing pH. (a) NaCl (b) NH4Cl (c) HCl (d)... Problem 91GQ: m-Nitrophenol, a weak acid, can be used as a pH indicator because it is yellow at pH above 8.6 and... Problem 92GQ: The butylammonium ion, C4H9NH3+, has a Ka of 2.3 1011. C4H9NH3+(aq) + H2O() H3O+(aq) + C4H9NH2(aq)... Problem 93GQ: The local anaesthetic novocaine is the hydrogen chloride salt of an organic base, procaine.... Problem 94GQ: Pyridine is weak organic base and readily forms a salt with hydrochloric acid.... Problem 95GQ: The base ethylamine (CH3CH2NH2) has a Kb of. A closely related base, ethanolamine(HOCH2CH2NH2), has... Problem 96GQ: Chloroacetic acid, ClCH2CO2H, is a moderately weak acid (Ka=1.40103). If you dissolve 94.5 mg of the... Problem 97GQ: Saccharin (HC7H4NO3S) is a weak acid with pKa = 2.32 at 25 C. It is used in the form of sodium... Problem 98GQ: Given the following solutions: (a) 0.1 M NH3 (b) 0.1 M Na2CO3 (c) 0.1 M NaCl (d) 0.1 M CH3CO2H (e)... Problem 99GQ: For each of the following salts, predict whether a 0.10 M solution has a pH less than, equal to, or... Problem 100GQ: Nicotine, C10H14N2, has two basic nitrogen atoms (Figure 16.12), and both can react with water.... Problem 101GQ Problem 102GQ: The equilibrium constant for the reaction of hydrochloric acid and ammonia is 1.8 109 (page 726).... Problem 103GQ: The equilibrium constant for the reaction of formic acid and sodium hydroxide is 1.8 1010 (page... Problem 104GQ: Calculate the pH of the solution that results from mixing 25.0 mL of 0.14 M formic acid and 50.0 mL... Problem 105GQ: To what volume should 1.00 102 mL of any weak acid, HA, with a concentration 0.20 M be diluted to... Problem 106GQ: The hydrogen phthalate ion, C8HsO4, is a weak acid with Ka = 3.91 106.... Problem 107GQ Problem 108GQ Problem 109IL Problem 110IL Problem 111IL Problem 112IL: A hydrogen atom in the organic base pyridine, C5H5N, can be substituted by various atoms or groups... Problem 113IL: Nicotinic acid, C6H5NO2, is found in minute amounts in all living cells, hut appreciable amounts... Problem 114IL Problem 115IL: Sulfanilic acid, which is used in making dyes, is made by reacting aniline with sulfuric acid. (a)... Problem 116IL: Amino acids are an important group of compounds. At low pH, both the carboxylic acid group (CO2H)... Problem 117SCQ: How can water be both a Brnsied base and a Lewis base? Can water be a Brnsted acid? A Lewis acid? Problem 118SCQ: The nickel(II) ion exists as [Ni(H2O)4]2+ in aqueous solution. Why is this solution acidic? As part... Problem 119SCQ: The halogens form three stable, weak acids, HOX. (a) Which is the strongest of these acids? (b)... Problem 120SCQ: The acidity of the oxoacids was described in Section 16.9, and a larger number of acids are listed... Problem 121SCQ: Perchloric acid behaves as an acid, even when it is dissolved in sulfuric acid. (a) Write a balanced... Problem 122SCQ: You purchase a bottle of water. On checking its pH, you find that it is not neutral, as you might... Problem 123SCQ Problem 124SCQ Problem 125SCQ Problem 127SCQ: Consider a salt of a weak base and a weak acid such as ammonium cyanide. Both the NH4+ and CN ions... format_list_bulleted

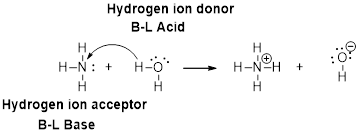
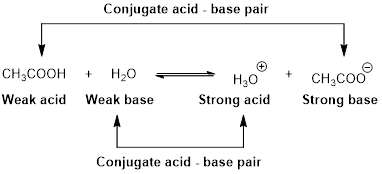
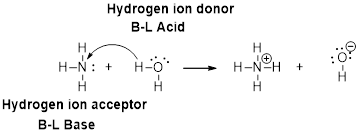

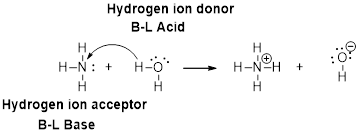

 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

