
(a)
Interpretation:
Chemical equation for the conversion of given
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
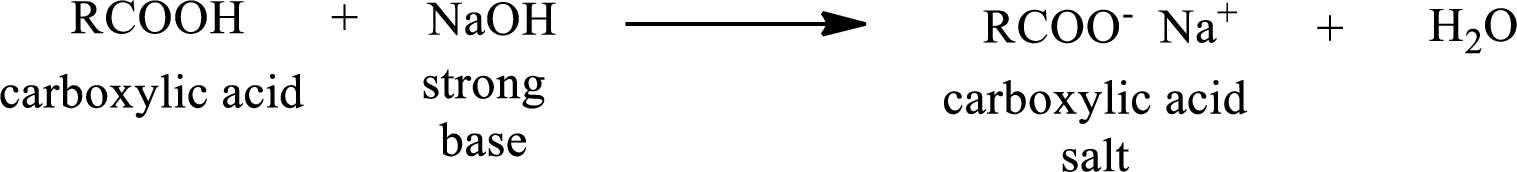
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
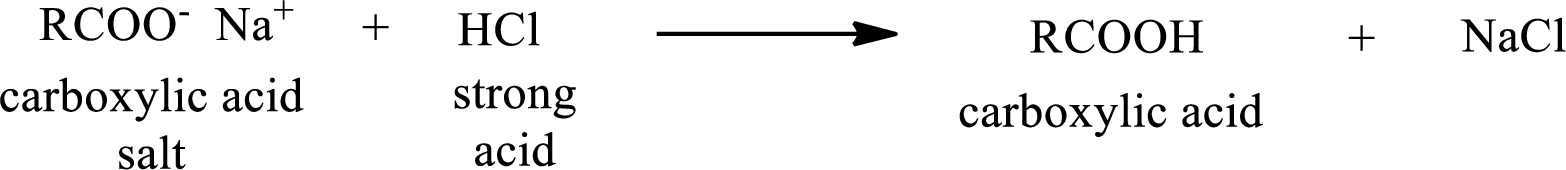
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(a)
Explanation of Solution
Given carboxylic acid salt is calcium propanoate. The structure of calcium propanoate can be given as shown below,
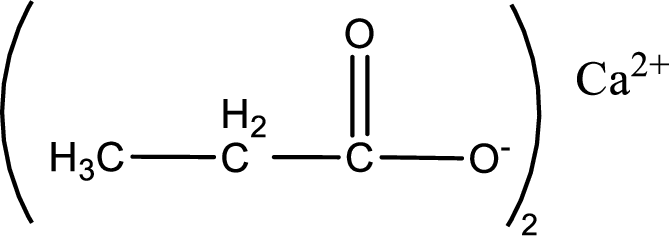
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
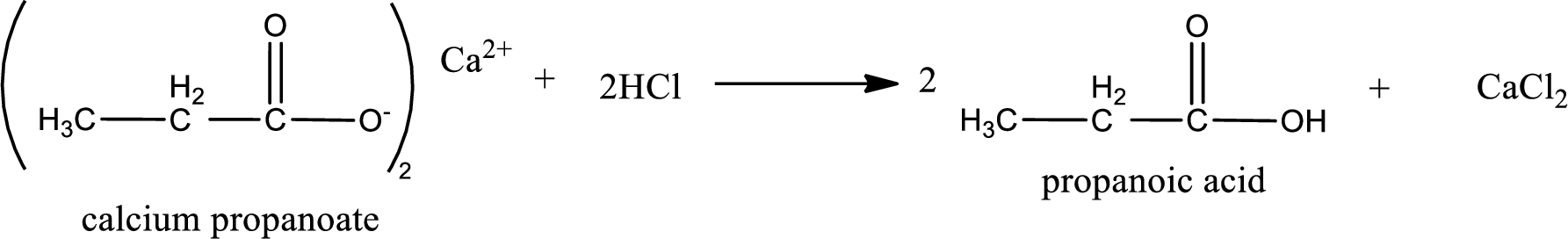
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
(b)
Interpretation:
Chemical equation for the conversion of given carboxylic acid salt to its parent carboxylic acid using
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
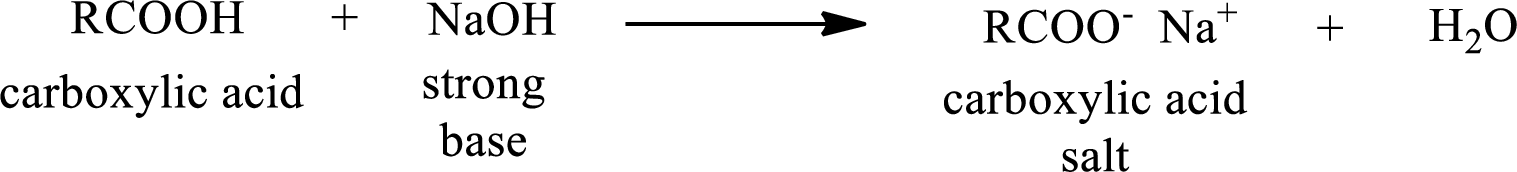
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
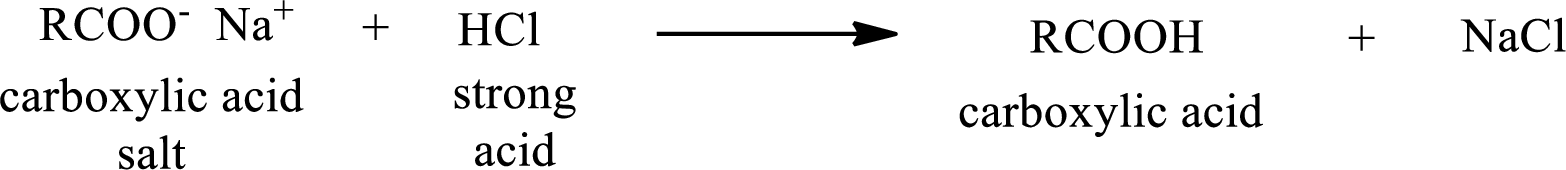
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(b)
Explanation of Solution
Given carboxylic acid salt is sodium lactate. The structure of sodium lactate can be given as shown below,
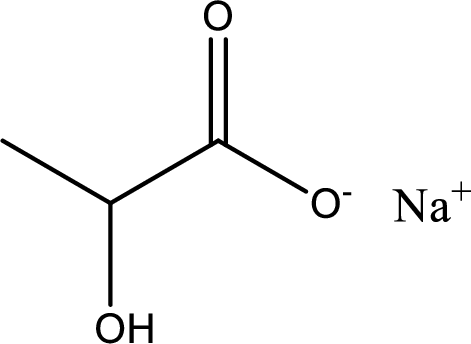
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
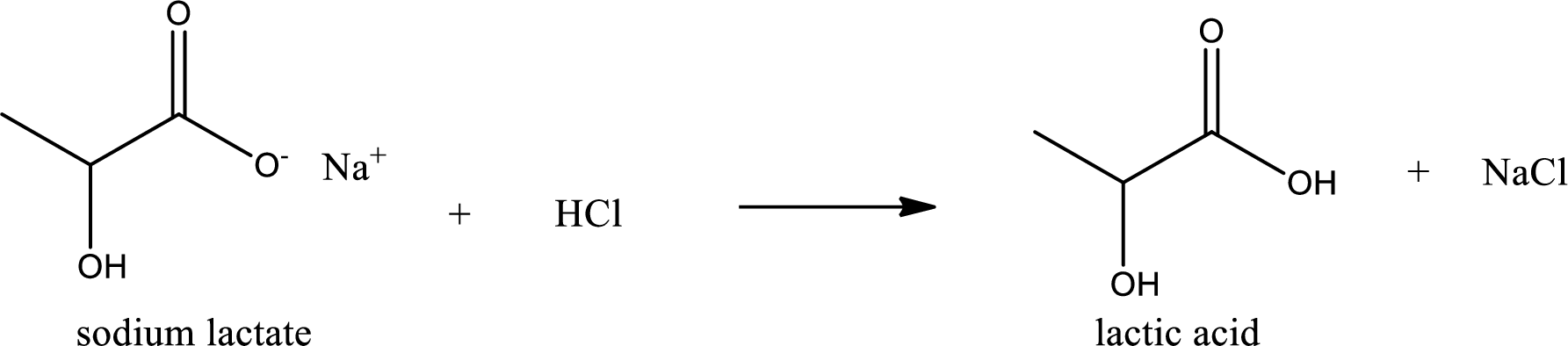
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
(c)
Interpretation:
Chemical equation for the conversion of given carboxylic acid salt to its parent carboxylic acid using
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
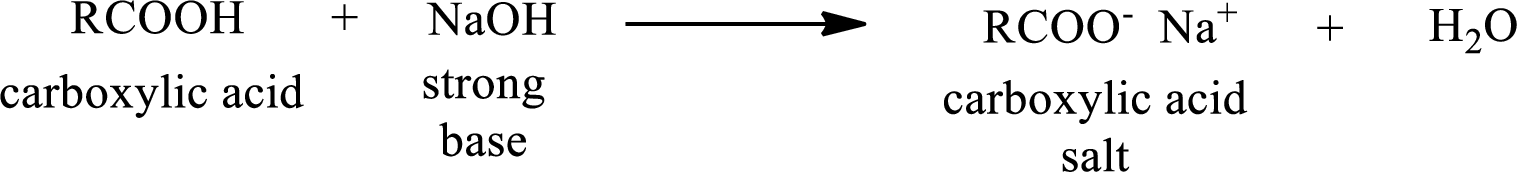
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
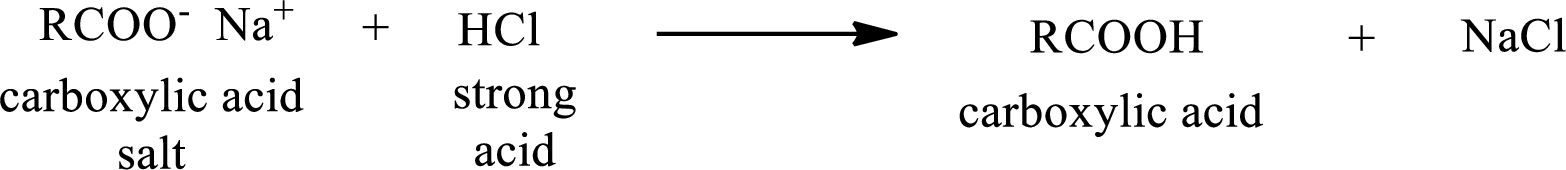
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(c)
Explanation of Solution
Given carboxylic acid salt is magnesium succinate. The structure of magnesium succinate can be given as shown below,
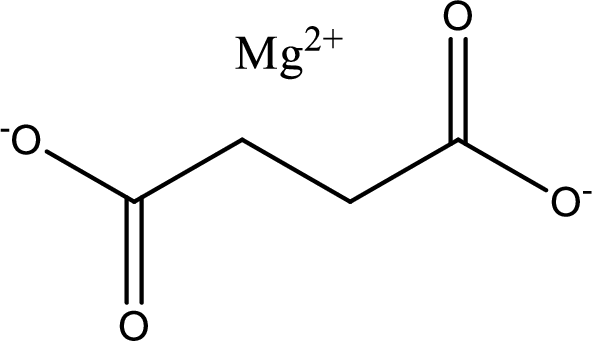
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
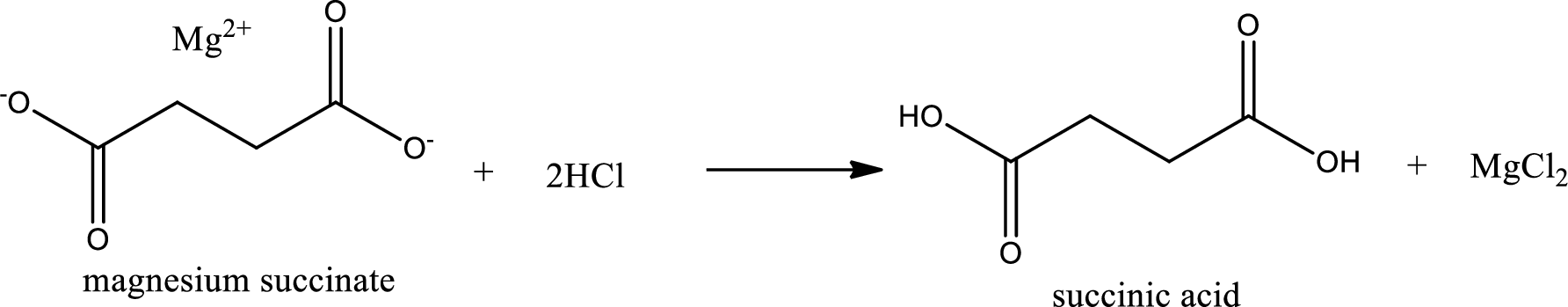
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
(d)
Interpretation:
Chemical equation for the conversion of given carboxylic acid salt to its parent carboxylic acid using
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
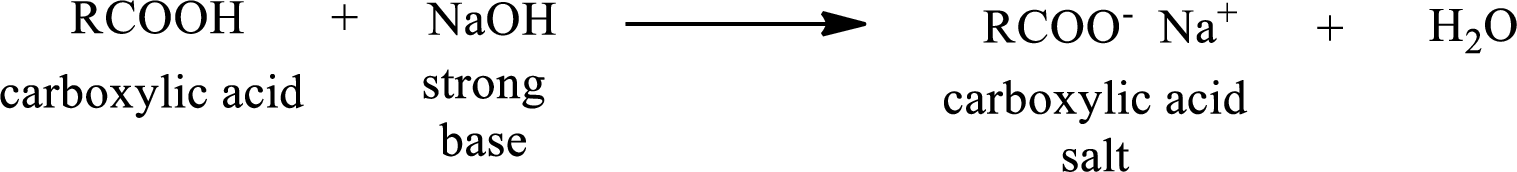
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
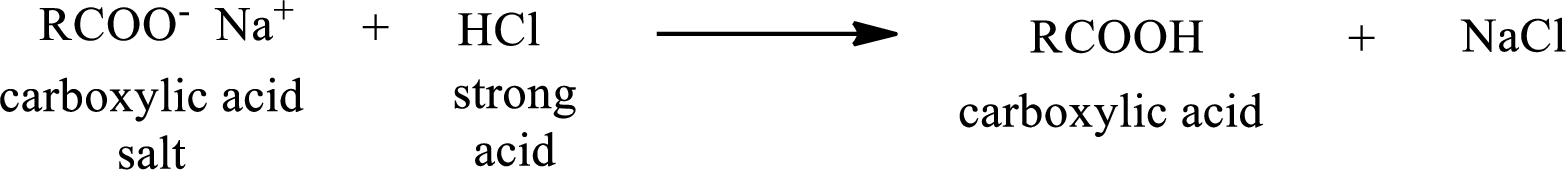
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(d)
Explanation of Solution
Given carboxylic acid salt is potassium benzoate. The structure of potassium benzoate can be given as shown below,
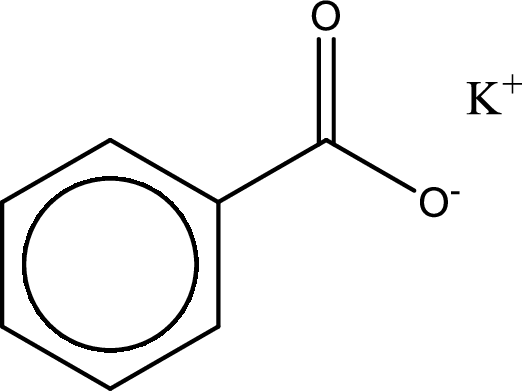
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
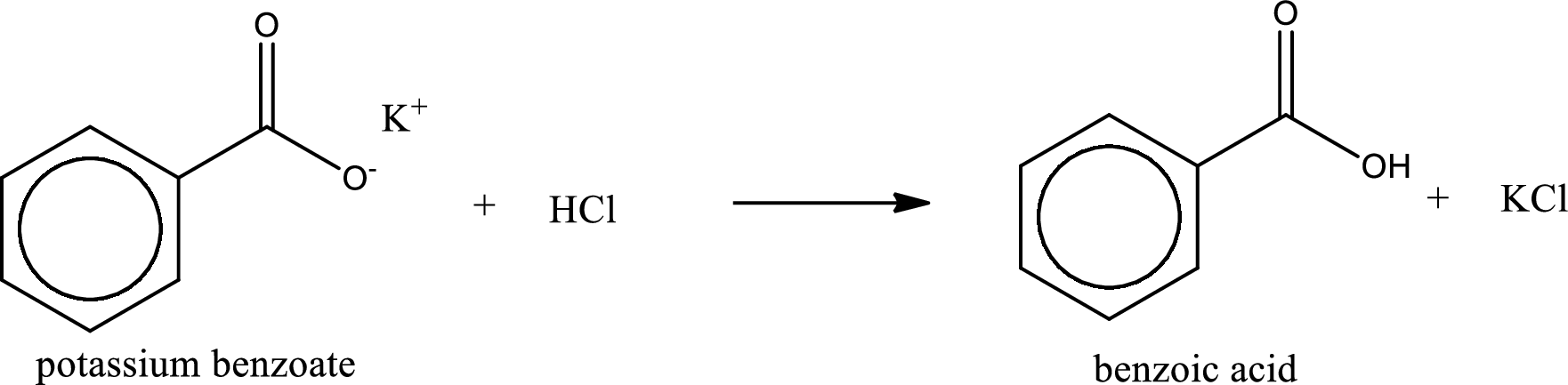
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
Want to see more full solutions like this?
Chapter 16 Solutions
General, Organic, and Biological Chemistry Seventh Edition
- If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forward
- Does Avogadro's number have units?arrow_forwardExplain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forward
- Indicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forward
- For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forwardWrite the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.arrow_forwardName the following using IUPAC.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





