
(a)
Interpretation:
The compounds mesitylene, toluene, and
Concept introduction:
The replacement of hydrogen atom attached to a carbon atom of electron-rich benzene ring by an electrophile is known as electrophilic aromatic substitution reaction. The rate of electrophilic aromatic substitution reaction depends on the substituted group on the aromatic ring. The ring deactivating group retards the electrophilic aromatic substitution reaction and ring activating group enhances the electrophilic aromatic substitution reaction.
Answer to Problem 16.44AP
The compounds mesitylene, toluene, and
Explanation of Solution
The structure of mesitylene, toluene, and
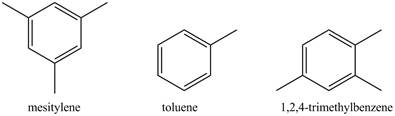
Figure 1
The reaction of any
The methyl group is electron-donating group. It activates the phenyl ring. The toluene has only one methyl group attached to it. Therefore, it will be least reactive towards
The order of reactivity toward nitration reaction is shown below.
The increasing order of reactivity towards toward
(b)
Interpretation:
The compounds chlorobenzene, benzene, and nitrobenzene are to be arranged in increasing order of increasing reactivity toward
Concept introduction:
The replacement of hydrogen atom attached to a carbon atom of electron-rich benzene ring by an electrophile is known as electrophilic aromatic substitution reaction. The rate of electrophilic aromatic substitution reaction depends on the substituted group on the aromatic ring. The ring deactivating group retards the electrophilic aromatic substitution reaction and ring activating group enhances the electrophilic aromatic substitution reaction.
Answer to Problem 16.44AP
The compounds chlorobenzene, benzene, and nitrobenzene are arranged in increasing order of increasing reactivity toward
Explanation of Solution
The structure of chlorobenzene, benzene, and nitrobenzene are shown below.
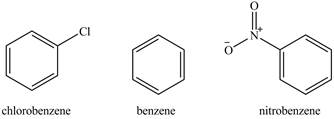
Figure 2
The reaction of any aromatic compound with
The nitro and chloro groups are electron-withdrawing groups. Therefore, the reactivity of the chlorobenzene and nitrobenzene will be less than that of benzene. The nitro group is stronger deactivating group than chloro group. Therefore, the order of reactivity toward nitration reaction is shown below.
The increasing order of reactivity towards toward
(c)
Interpretation:
The compounds
Concept introduction:
The replacement of hydrogen atom attached to a carbon atom of electron-rich benzene ring by an electrophile is known as electrophilic aromatic substitution reaction. The rate of electrophilic aromatic substitution reaction depends on the substituted group on the aromatic ring. The ring deactivating group retards the electrophilic aromatic substitution reaction and ring activating group enhances the electrophilic aromatic substitution reaction.
Answer to Problem 16.44AP
The compounds
Explanation of Solution
The structure of
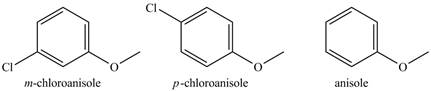
Figure 3
The reaction of any aromatic compound with
The methoxy group and chloro groups are ortho and para directing groups. The methoxy group is electron releasing group and chloro group is electron-withdrawing group.
Therefore, the reactivity of anisole will be highest among the rest of the compound toward
The order of reactivity toward nitration reaction is shown below.
The increasing order of reactivity towards toward
(d)
Interpretation:
The compounds acetophenone,
Concept introduction:
The replacement of hydrogen atom attached to a carbon atom of electron-rich benzene ring by an electrophile is known as electrophilic aromatic substitution reaction. The rate of electrophilic aromatic substitution reaction depends on the substituted group on the aromatic ring. The ring deactivating group retards the electrophilic aromatic substitution reaction and ring activating group enhances the electrophilic aromatic substitution reaction.
Answer to Problem 16.44AP
The compounds acetophenone,
Explanation of Solution
The structure of acetophenone,
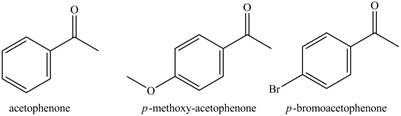
Figure 4
The reaction of any aromatic compound with
The acetyl group and bromo group is electron-withdrawing groups and methoxy group is electron releasing group. Acetophenone has a ring activating group attached on it. Therefore, it is most reactive toward nitration reaction among the rest of the compound. The compound
The increasing order of reactivity towards toward
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry Study Guide and Solutions
- V Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forwardComplete the mechanismarrow_forward
- 8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forwardThe grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





