
Concept explainers
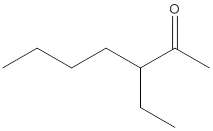
(a)
Interpretation:
The name of the given ketone is to be determined.
Concept Introduction:
While naming the
(b)
Interpretation:
The name of the given ketone is to be determined.
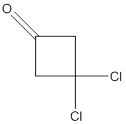
Concept Introduction:
While naming the ketones as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-one in the end of the name. Firstly, one will find the longest chain that contains the -C=O group and then change the -e ending of the parent alkane chain to -one suffix. Then, the numbering of the chain or the ring is done in such a way that the carbonyl group is given the lowest possible number. Thereafter, apply all other rules of nomenclature as usual.
(c)
Interpretation:
The name of the given ketone is to be determined.
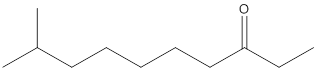
Concept Introduction:
While naming the ketones as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-one in the end of the name. Firstly, one will find the longest chain that contains the -C=O group and then change the -e ending of the parent alkane chain to -one suffix. Then, the numbering of the chain or the ring is done in such a way that the carbonyl group is given the lowest possible number. Thereafter, apply all other rules of nomenclature as usual.
(d)
Interpretation:
The name of the given ketone is to be determined.
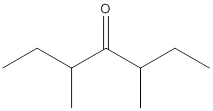
Concept Introduction:
While naming the ketones as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-one in the end of the name. Firstly, one will find the longest chain that contains the -C=O group and then change the -e ending of the parent alkane chain to -one suffix. Then, the numbering of the chain or the ring is done in such a way that the carbonyl group is given the lowest possible number. Thereafter, apply all other rules of nomenclature as usual.
(e)
Interpretation:
The name of the given ketone is to be determined.
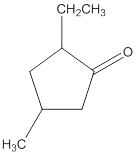
Concept Introduction:
While naming the ketones as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-one in the end of the name. Firstly, one will find the longest chain that contains the -C=O group and then change the -e ending of the parent alkane chain to -one suffix. Then, the numbering of the chain or the ring is done in such a way that the carbonyl group is given the lowest possible number. Thereafter, apply all other rules of nomenclature as usual.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
General, Organic, & Biological Chemistry
- Indicate the products of the reaction between CH3COCH2COONa (Sodium acetoacetate) and BrCH2COOC2H5arrow_forwardIndicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forward
- Primary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forwardFormulate the reaction: Naphthalene with CrO3 in acetic acid at 25ºCarrow_forwardComplete the reaction hand written pleasearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning




