
Concept explainers
(a)
Interpretation:
The products should be identified by the reaction of benzaldehyde with
Concept Introduction:
Addition of H2gas to a multiple bond is known as hydrogenation. In the presence of palladium metal as the catalyst H2 molecules react with
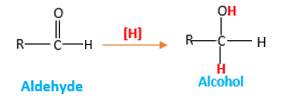
Answer to Problem 16.85P
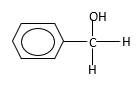
Explanation of Solution
When an aldehyde reacts with H2 gas in the presence of palladium metal resulting product is an alcohol of the initial aldehyde molecule. Palladium metal act as a catalyst to the reaction that provides a surface to bind both the H2 and carbonyl compound which reduce the activation energy of the reaction.
Hydrogen atoms in the alcohol molecule shown below, which are indicated in red color are the added H during the hydrogenation reaction.
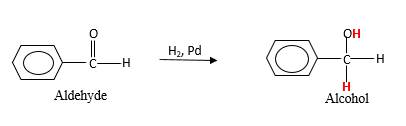
(b)
Interpretation:
The products should be identified by the reaction of benzaldehyde with
Concept Introduction:
Addition of an O atom in to a molecule is known as oxidation. The hydrogen atom directly connected to the carbonyl C in an aldehyde will oxidized in the presence of an oxidizing agent such as
Oxygen atom in the carboxylicacid molecule shown below, which is indicated in red color is the added O during the oxygenation reaction.
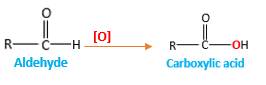
Answer to Problem 16.85P
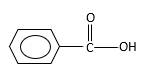
Explanation of Solution
When an aldehyde reacts with
(c)
Interpretation:
The products should be identified by the reaction of benzaldehyde with
Concept Introduction:
Addition of an O atom in to a molecule is known as oxidation. The hydrogen atom directly connected to the carbonyl C in an aldehyde will oxidized in the presence of an oxidizing agent such as (
Oxygen atom in the carboxylic acid molecule shown below, which is indicated in red color is the added O during the oxygenation reaction.
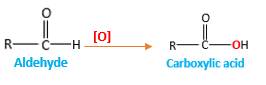
Answer to Problem 16.85P
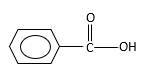
Explanation of Solution
When an aldehyde reacts with
Aldehyde's
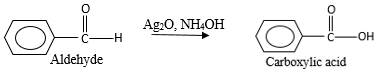
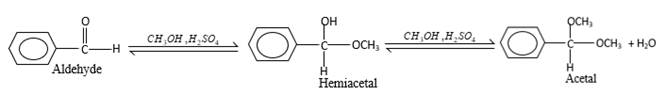
(d)
Interpretation:
The products should be identified by the reaction of benzaldehyde with
Concept Introduction:
In the presence of alcohol in the acidic medium, aldehydes undergo addition reactions and give acetal in two steps.
Hydrogen atom and CH3 groups in the acetal and hemiacetal molecules shown below, which are indicated in red color are the added molecules during the reaction.
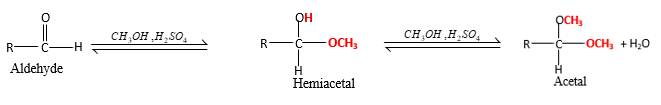
Answer to Problem 16.85P
Explanation of Solution
In the presence of alcohol in the acidic medium, aldehydes undergo addition reactions and give acetal in two steps. In the first step aldehydes form hemiacetals and during the second step it converts to an acetal molecule of the respective aldehyde molecule.
Addition of one molecule of alcohol in to an aldehyde forms a hemiacetal, one bond of the
(e)
Interpretation:
The products should be identified by the reaction of benzaldehyde with
Concept Introduction:
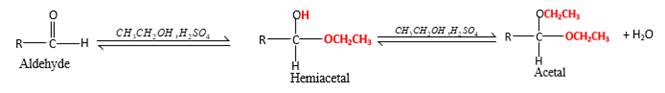
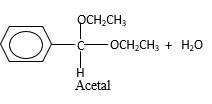
In the presence of alcohol in the acidic medium, aldehydes undergo addition reactions and give acetal in two steps.
Hydrogen atom and CH2CH3 groups in the acetal and hemiacetal molecules shown below, which are indicated in red color are the added molecules during the reaction.
Answer to Problem 16.85P
Explanation of Solution
In the presence of alcohol in the acidic medium, aldehydes undergo addition reactions and give acetal in two steps. In the first step aldehydes form hemiacetals and during the second step it converts to an acetal molecule of the respective aldehyde molecule.
Addition of one molecule of alcohol in to an aldehyde forms a hemiacetal, one bond of the
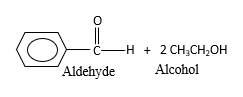
(f)
Interpretation:
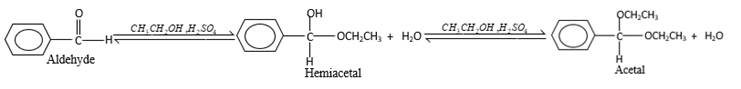
The products should be identified by the reaction of
Concept Introduction:
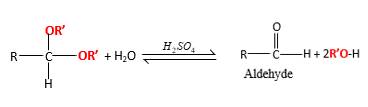
In the presence of water and acid, acetals undergo hydrolysis reaction and produce aldehydes.
OR' groups in the acetal molecule shown below, which are indicated in red color are the molecules which becomes alcohol molecules during the hydrolysis.
Answer to Problem 16.85P
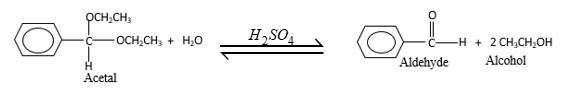
Explanation of Solution
Acetals are stable molecules, but their bonds can cleave by a reaction with water and produce aldehydes.
In the acetal molecule, two bonds of the
Want to see more full solutions like this?
Chapter 16 Solutions
General, Organic, & Biological Chemistry
- Indicate the products of the reaction between CH3COCH2COONa (Sodium acetoacetate) and BrCH2COOC2H5arrow_forwardIndicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forward
- Primary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forwardFormulate the reaction: Naphthalene with CrO3 in acetic acid at 25ºCarrow_forwardComplete the reaction hand written pleasearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





