
CHEMISTRY-TEXT
8th Edition
ISBN: 9780134856230
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 15.47CP
The following pictures represent equilibrium mixtures at 325 Kand 350 K for a reaction involving A atoms (red), B atoms (blue),and AB molecules:
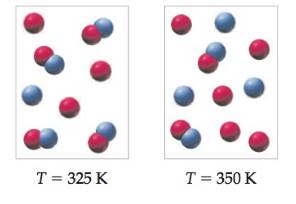
(a) Write a balanced equation for the reaction that occurs onraising the temperature.
(b) Is the reaction exothermic or endothermic? Explain usingLe Châtelier’s principle.
(c) if the volume of the container is increased, will the number ofA atoms increase, decrease, or remain the same? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please predict the products for each of the
following reactions.
Clearly show the regiochemistry (Markovnikov
vs anti-Markovnikov) and stereochemistry
(syn- vs anti- or both).
If a mixture of enantiomers is formed, please
draw all the enantiomers.
Hint: In this case you must choose the best
answer to demonstrate the stereochemistry of
H2 addition.
1.03
2. (CH3)2S
BIZ
CH₂OH
2. DMS
KMnO4, NaOH
ΖΗ
Pd or Pt (catalyst)
HBr
20 1
HBr
ROOR (peroxide)
HO
H-SO
HC
12 11 10
BH, THE
2. H2O2, NaOH
Brz
cold
HI
19
18
17
16
MCPBA
15
14
13
A
Br
H₂O
BH3⚫THF
Brz
EtOH
Pd or Ni (catalyst)
D₂ (deuterium)
1. Os04
2. H2O2
CH3CO3H
(peroxyacid)
1. MCPBA
2. H₂O*
H
B
+
H
H
H
"H
C
H
H
D
Explain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.
Explain the importance of having a sampling plan with respect to food analysis.
Explain the importance of having a sampling plan with respect to food analysis. Provide examples.
Chapter 15 Solutions
CHEMISTRY-TEXT
Ch. 15 - The oxidation of sulfur dioxide to give...Ch. 15 - APPLY 14.2 Nitrogen dioxide, a pollutant that...Ch. 15 - The following equilibrium concentrations were...Ch. 15 - APPLY 14.4 Lactic acid, which builds up in muscle...Ch. 15 - Conceptual PRACTICE 14.5 The following pictures...Ch. 15 - Conceptual APPLY 14.6 The equilibrium constant...Ch. 15 - PRACTICE 14.7 In the industrial synthesis of...Ch. 15 - APPLY 14.8 At 25 °C, Kp = 25 for the reaction...Ch. 15 - Nitric oxide reacts with oxygen to give nitrogen...Ch. 15 - APPLY 14.10For the reaction...
Ch. 15 - Write the equilibrium constant expression (Kp)...Ch. 15 - APPLY 14.12 Magnesium hydroxide is the active...Ch. 15 - When wine spoils, ethanol is oxidized to acetic...Ch. 15 - The value of K for the dissociation reaction...Ch. 15 - The equilibrium constant K. for the reaction...Ch. 15 - Conceptual APPLY 14.16 The reaction A2 + B2 2...Ch. 15 - PRACTICE 14.17 The H2/CO ratio in mixtures of...Ch. 15 - APPLY 14.18 Calculate the equilibrium...Ch. 15 - PRACTICE 14.19 Calculate the equilibrium...Ch. 15 - APPLY 14.20 Calculate the equilibrium...Ch. 15 - Calculate the equilibrium concentrations of...Ch. 15 - Calculate the equilibrium concentrations of...Ch. 15 - The equilibrium constant Kp , for the reaction...Ch. 15 - The equilibrium constant Kp , for the reaction...Ch. 15 - Consider the equilibrium for the water—gas shift...Ch. 15 - Solid particles that form in the kidney are called...Ch. 15 - Does the number of moles of products...Ch. 15 - CONCEPTUAL APPLY 15.28 The following picture...Ch. 15 - When air is heated at very high temperatures in an...Ch. 15 - Ethyl acetate, a solvent used in many...Ch. 15 - The following pictures represent the composition...Ch. 15 - The following picture represents an equilibrium...Ch. 15 - Nitric oxide emitted from the engines of...Ch. 15 - The energy profile of the reaction krKf is shown....Ch. 15 - Refer to Figure 15.16 to answer the following...Ch. 15 - Prob. 15.36PCh. 15 - Prob. 15.37PCh. 15 - Prob. 15.38PCh. 15 - Prob. 15.39PCh. 15 - Prob. 15.40PCh. 15 - Prob. 15.41CPCh. 15 - The following pictures represent the equilibrium...Ch. 15 - The reaction A2+BA+AB has an equilibrium constant...Ch. 15 - Prob. 15.44CPCh. 15 - Prob. 15.45CPCh. 15 - Prob. 15.46CPCh. 15 - The following pictures represent equilibrium...Ch. 15 - Prob. 15.48CPCh. 15 - Prob. 15.49CPCh. 15 - Prob. 15.50CPCh. 15 - Prob. 15.51CPCh. 15 - Prob. 15.52SPCh. 15 - Identify the true statement about the...Ch. 15 - Prob. 15.54SPCh. 15 - Prob. 15.55SPCh. 15 - For each of the following equilibria, write the...Ch. 15 - Prob. 15.57SPCh. 15 - Prob. 15.58SPCh. 15 - Prob. 15.59SPCh. 15 - For each of the following equilibria, write the...Ch. 15 - Prob. 15.61SPCh. 15 - The reaction 2AsH3(g)As2(g)+3H2(g) has Kp = 7.2107...Ch. 15 - Prob. 15.63SPCh. 15 - Calculate the value of the equilibrium constant at...Ch. 15 - Prob. 15.65SPCh. 15 - An equilibrium mixture of PCI5, PCi3, and Cl2 at a...Ch. 15 - The partial pressures in an equilibrium mixture of...Ch. 15 - At 298 K, Kc is 2.2105 for the reaction F (g) + O2...Ch. 15 - At 298 K, Kp is 1.6106 for the reaction...Ch. 15 - Prob. 15.70SPCh. 15 - Prob. 15.71SPCh. 15 - Prob. 15.72SPCh. 15 - Prob. 15.73SPCh. 15 - For each of the following equilibria, write the...Ch. 15 - For each of the following equilibria, write the...Ch. 15 - When the following reactions come to equilibrium,...Ch. 15 - Prob. 15.77SPCh. 15 - A chemical engineer is studying reactions to...Ch. 15 - Prob. 15.79SPCh. 15 - At 1400 K, Kc = 103 for the reaction...Ch. 15 - The first step in the industrial synthesis of...Ch. 15 - Phosphine (PH3) decomposes at elevated...Ch. 15 - Prob. 15.83SPCh. 15 - Calculate the equilibrium concentrations of N2O4...Ch. 15 - Calculate the equilibrium concentrations at 25 °C...Ch. 15 - A sample of HI (9.30 103 mol) was placed in an...Ch. 15 - The industrial solvent ethyl acetate is produced...Ch. 15 - Prob. 15.88SPCh. 15 - Prob. 15.89SPCh. 15 - The following reaction, which has Kc = 0.145 at...Ch. 15 - An equilibrium mixture of N2, H2, and NH1 at 700 K...Ch. 15 - An equilibrium mixture of O2 , SO2 , and SO4...Ch. 15 - The air pollutant NO is produced in automobile...Ch. 15 - Prob. 15.94SPCh. 15 - Prob. 15.95SPCh. 15 - Prob. 15.96SPCh. 15 - Prob. 15.97SPCh. 15 - Prob. 15.98SPCh. 15 - The reaction of iron (III) oxide with carbon...Ch. 15 - The equilibrium concentrations in a gas mixture at...Ch. 15 - Prob. 15.101SPCh. 15 - Prob. 15.102SPCh. 15 - Prob. 15.103SPCh. 15 - Prob. 15.104SPCh. 15 - Prob. 15.105SPCh. 15 - At 100 °C, K = 4.72 for the reaction...Ch. 15 - At 25 °C, Kc = 216 for the reaction...Ch. 15 - At 500 °C, F2 , gas is stable and does not...Ch. 15 - Prob. 15.109SPCh. 15 - Phosgene ( COCl2 ) is a toxic gas that damages the...Ch. 15 - Prob. 15.111SPCh. 15 - At 45 °C, Kc = 0.6 19 for the reaction...Ch. 15 - Prob. 15.113SPCh. 15 - Prob. 15.114SPCh. 15 - Prob. 15.115SPCh. 15 - Prob. 15.116SPCh. 15 - Prob. 15.117SPCh. 15 - Consider the following equilibrium:...Ch. 15 - Will the concentration of NO2 increase, decrease,...Ch. 15 - When each of the following equilibria is disturbed...Ch. 15 - Prob. 15.121SPCh. 15 - For the water-gas shift reaction...Ch. 15 - Prob. 15.123SPCh. 15 - Consider the exothermic reaction...Ch. 15 - Prob. 15.125SPCh. 15 - Methanol ( CH3OH ) is manufactured by the reaction...Ch. 15 - In the gas phase at 400°C, isopropyl alcohol...Ch. 15 - The following reaction is important in gold...Ch. 15 - Prob. 15.129SPCh. 15 - The equilibrium constant Kp for the reaction...Ch. 15 - Prob. 15.131SPCh. 15 - Prob. 15.132SPCh. 15 - Consider the following gas-phase reaction:...Ch. 15 - Prob. 15.134SPCh. 15 - Prob. 15.135SPCh. 15 - Prob. 15.136SPCh. 15 - Prob. 15.137SPCh. 15 - Prob. 15.138SPCh. 15 - Prob. 15.139SPCh. 15 - Prob. 15.140SPCh. 15 - Prob. 15.141SPCh. 15 - Prob. 15.142SPCh. 15 - Prob. 15.143SPCh. 15 - Prob. 15.144MPCh. 15 - Prob. 15.145MPCh. 15 - Refining petroleum involves cracking large...Ch. 15 - At 1000 K, Kp = 2.1 106 and H ° = -107.7 kJ for...Ch. 15 - Consider the gas-phase decomposition of...Ch. 15 - Prob. 15.149MPCh. 15 - Prob. 15.150MPCh. 15 - Prob. 15.151MPCh. 15 - Prob. 15.152MPCh. 15 - Prob. 15.153MPCh. 15 - Prob. 15.154MPCh. 15 - A 125.4 g quantity of water and an equal molar...Ch. 15 - Prob. 15.156MPCh. 15 - Prob. 15.157MPCh. 15 - Prob. 15.158MPCh. 15 - Prob. 15.159MPCh. 15 - Ozone is unstable with respect to decomposition to...Ch. 15 - Prob. 15.161MPCh. 15 - For the decomposition reaction...Ch. 15 - Prob. 15.163MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forward
- Briefly state the electrocapillary equation for ideally polarized electrodes.arrow_forwardWhat is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forward
- The molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY