
Concept explainers
(a)
Interpretation: The Lewis structure, molecular structure and expected hybrid orbitals on central atom, polarity of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
The polarity of molecule is determined with the help of presence of polar bond and symmetrical geometry.
(a)
Answer to Problem 23E
Molecular structure = Tetrahedral
Polarity = Non-polar
Hybridization = sp3
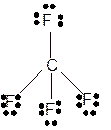
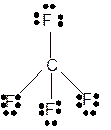
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
(b)
Interpretation: The Lewis structure, molecular structure and expected hybrid orbitals on central atom, polarity of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
The polarity of molecule is determined with the help of presence of polar bond and symmetrical geometry.
(b)
Answer to Problem 23E
Molecular structure = pyramid
Polarity = polar
Hybridization = sp3
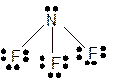
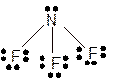
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
(c)
Interpretation: The Lewis structure, molecular structure and expected hybrid orbitals on central atom, polarity of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
The polarity of molecule is determined with the help of presence of polar bond and symmetrical geometry.
(c)
Answer to Problem 23E
Molecular structure = bent
Polarity = polar
Hybridization = sp3
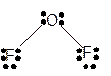
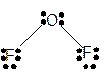
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
(d)
Interpretation: The Lewis structure, molecular structure and expected hybrid orbitals on central atom, polarity of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
The polarity of molecule is determined with the help of presence of polar bond and symmetrical geometry.
(d)
Answer to Problem 23E
Molecular structure = trigonal planer
Polarity = non-polar
Hybridization = sp2
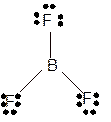
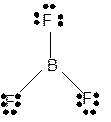
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
(e)
Interpretation: The Lewis structure, molecular structure and expected hybrid orbitals on central atom, polarity of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
The polarity of molecule is determined with the help of presence of polar bond and symmetrical geometry.
(e)
Answer to Problem 23E
Molecular structure = linear
Polarity = non-polar
Hybridization = sp
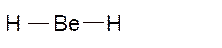
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
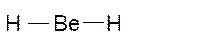
(f)
Interpretation: The Lewis structure, molecular structure and expected hybrid orbitals on central atom, polarity of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
The polarity of molecule is determined with the help of presence of polar bond and symmetrical geometry.
(f)
Answer to Problem 23E
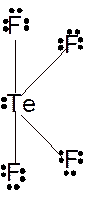
Molecular structure = see-saw
Polarity = polar
Hybridization = sp3d
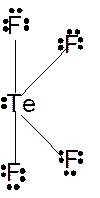
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
(g)
Interpretation: The Lewis structure, molecular structure and expected hybrid orbitals on central atom, polarity of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
The polarity of molecule is determined with the help of presence of polar bond and symmetrical geometry.
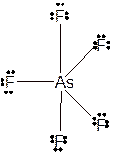
(g)
Answer to Problem 23E
Molecular structure = trigonal bipyramid
Polarity = non-polar
Hybridization = sp3d
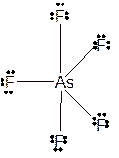
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
(h)
Interpretation: The Lewis structure, molecular structure and expected hybrid orbitals on central atom, polarity of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
The polarity of molecule is determined with the help of presence of polar bond and symmetrical geometry.
(h)
Answer to Problem 23E
Molecular structure = linear
Polarity = non-polar
Hybridization = sp3d
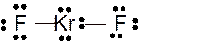
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
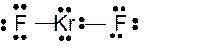
(i)
Interpretation: The Lewis structure, molecular structure and expected hybrid orbitals on central atom, polarity of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
The polarity of molecule is determined with the help of presence of polar bond and symmetrical geometry.
(i)
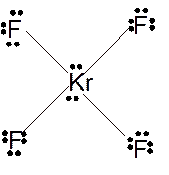
Answer to Problem 23E
Molecular structure = square planer
Polarity = non-polar
Hybridization = sp3d2
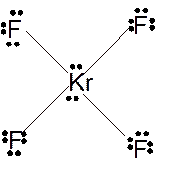
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
(j)
Interpretation: The Lewis structure, molecular structure and expected hybrid orbitals on central atom, polarity of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
The polarity of molecule is determined with the help of presence of polar bond and symmetrical geometry.
(j)
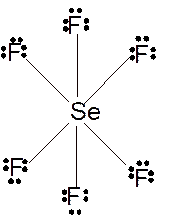
Answer to Problem 23E
Molecular structure = octahedral
Polarity = non-polar
Hybridization = sp3d2
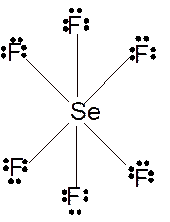
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
(k)
Interpretation: The Lewis structure, molecular structure and expected hybrid orbitals on central atom, polarity of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
The polarity of molecule is determined with the help of presence of polar bond and symmetrical geometry.
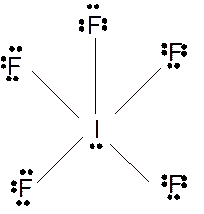
(k)
Answer to Problem 23E
Molecular structure = square pyramid
Polarity = polar
Hybridization = sp3d2
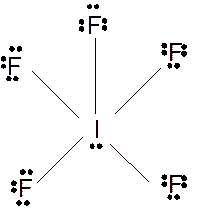
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
(l)
Interpretation: The Lewis structure, molecular structure and expected hybrid orbitals on central atom, polarity of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
The polarity of molecule is determined with the help of presence of polar bond and symmetrical geometry.
(l)
Answer to Problem 23E
Molecular structure = T-shape
Polarity = polar
Hybridization = sp3d

Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for

Want to see more full solutions like this?
Chapter 14 Solutions
Chemical Principles
- 20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forwardGiven the attached data, provide the drawing for the corresponding structure.arrow_forwardno Ai walkthroughsarrow_forward

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





