
(a)
Interpretation:
The hybrid orbitals should be predicted which is used by sulfur atom(s) in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct atoms to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
When the atomic orbitals overlap with each other in a region of high electron density, then molecular orbitals are formed. Overlapping of atomic orbitals determines the efficiency of interaction between atomic orbitals. Energy of bond molecular orbitals is less than the nonbonding molecular orbitals.
(a)
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
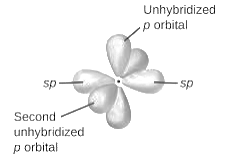
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an tom to produce two new equal orbitals. This hybridization takes place in linear molecules.
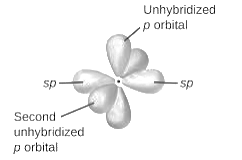
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an tom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an tom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
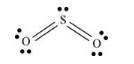
The given molecule is
Number of valence electrons in sulfur = 6
Number of valence electrons in oxygen = 6
Total number of valence electrons =
The bonds present between sulfur atom and oxygen atom is sigma and pi bonds and also one lone pair of electrons are present on sulfur atom which pushes the oxygen atom down. Thus, geometry of
Electronic configuration of sulfur is
Electronic configuration of oxygen is
The sulfur atom uses one
Lewis structure of
(b)
Interpretation:
The hybrid orbitals should be predicted which is used by sulfur atom(s) in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct atoms to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
When the atomic orbitals overlap with each other in a region of high electron density, then molecular orbitals are formed. Overlapping of atomic orbitals determines the efficiency of interaction between atomic orbitals. Energy of bond molecular orbitals is less than the nonbonding molecular orbitals.
(b)
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an tom to produce two new equal orbitals. This hybridization takes place in linear molecules.
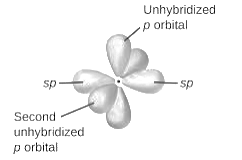
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an tom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an tom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
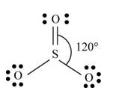
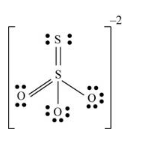
The given molecule is
Number of valence electrons in sulfur = 6
Number of valence electrons in oxygen = 6
Total number of valence electrons =
The bonds present between sulfur atom and oxygen atom is sigma and pi bonds. There are three bond pairs of electrons surrounding the sulfur atom. The oxygen atom which forms double bond with sulfur atom has two lone pairs and remaining two oxygen atoms has three lone pair of electrons.
Thus, geometry of
Electronic configuration of sulfur is
Electronic configuration of oxygen is
The sulfur atom form double bond which each oxygen atom implies one s and two p orbitals are mix with each other to form sp2 hybridization.
Lewis structure of
(c)
Interpretation:
The hybrid orbitals should be predicted which is used by sulfur atom(s) in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct atoms to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
When the atomic orbitals overlap with each other in a region of high electron density, then molecular orbitals are formed. Overlapping of atomic orbitals determines the efficiency of interaction between atomic orbitals. Energy of bond molecular orbitals is less than the nonbonding molecular orbitals.
(c)
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an tom to produce two new equal orbitals. This hybridization takes place in linear molecules.
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an tom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an tom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
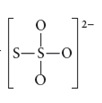
The given molecule is
Number of valence electrons in sulfur = 6
Number of valence electrons in oxygen = 6
According to structure of
Total number of valence electrons =
The bonds present between sulfur atom and sulfur atom and one oxygen atom is pi bond and remaining two oxygen atoms are linked by sigma bond.
Thus, geometry of
Electronic configuration of sulfur is
Electronic configuration of oxygen is
The central sulfur atom uses one s-orbital and three p orbitals and thus hybridization of central sulfur atom is sp3
Lewis structure of
(d)
Interpretation:
The hybrid orbitals should be predicted which is used by sulfur atom(s) in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct atoms to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
When the atomic orbitals overlap with each other in a region of high electron density, then molecular orbitals are formed. Overlapping of atomic orbitals determines the efficiency of interaction between atomic orbitals. Energy of bond molecular orbitals is less than the nonbonding molecular orbitals.
(d)
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an tom to produce two new equal orbitals. This hybridization takes place in linear molecules.
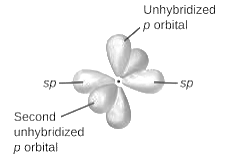
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an tom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an tom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
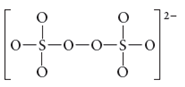
The given molecule is
Number of valence electrons in sulfur = 6
Number of valence electrons in oxygen = 6
There are eight oxygen atoms and two sulfur atoms are present in the molecule and charge on the molecule is equal to -2.
Total number of valence electrons =
Here, each sulfur atom is linked with four oxygen atoms, two oxygen atoms are double bonded and two oxygen atoms are single bonded.
The two oxygen atoms present in the center of the structure are bonded by single bond. By bonding in this way, each atom completes its octet.
Thus, geometry of
Electronic configuration of sulfur is
Electronic configuration of oxygen is
Both sulfur atoms uses one s-orbital and three p orbitals and thus hybridization of both sulfur atoms is sp3
Lewis structure of
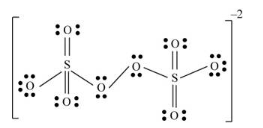
(e)
Interpretation:
The hybrid orbitals should be predicted which is used by sulfur atom(s) in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct atoms to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
When the atomic orbitals overlap with each other in a region of high electron density, then molecular orbitals are formed. Overlapping of atomic orbitals determines the efficiency of interaction between atomic orbitals. Energy of bond molecular orbitals is less than the nonbonding molecular orbitals.
(e)
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an tom to produce two new equal orbitals. This hybridization takes place in linear molecules.
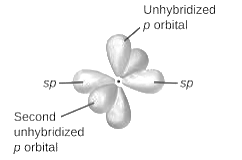
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an tom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an tom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
The given molecule is
Number of valence electrons in sulfur = 6
Number of valence electrons in oxygen = 6
There are three oxygen atoms and one sulfur atom present in the molecule and charge on the molecule is equal to -2.
Total number of valence electrons =
Here, sulfur atom is linked with three oxygen atoms where two oxygen atoms are single bonded with sulfur and one oxygen atom is double bonded with sulfur. By bonding in this way, each atom completes its octet.
Thus, geometry of
Electronic configuration of sulfur is
Electronic configuration of oxygen is
Sulfur atom uses one s-orbital and three p orbitals and thus hybridization of sulfur atom is sp3
Lewis structure of
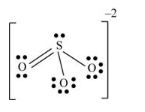
(f)
Interpretation:
The hybrid orbitals should be predicted which is used by sulfur atom(s) in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct atoms to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
When the atomic orbitals overlap with each other in a region of high electron density, then molecular orbitals are formed. Overlapping of atomic orbitals determines the efficiency of interaction between atomic orbitals. Energy of bond molecular orbitals is less than the nonbonding molecular orbitals.
(f)
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an tom to produce two new equal orbitals. This hybridization takes place in linear molecules.
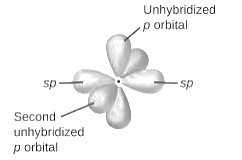
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an tom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an tom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
The given molecule is
Number of valence electrons in sulfur = 6
Number of valence electrons in oxygen = 6
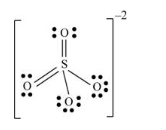
There are four oxygen atoms and one sulfur atom present in the molecule and charge on the molecule is equal to -2.
Total number of valence electrons =
Here, sulfur atom is linked with four oxygen atoms where two oxygen atoms are single bonded with sulfur and two oxygen atoms are double bonded with sulfur. By bonding in this way, each atom completes its octet.
Thus, geometry of
Electronic configuration of sulfur is
Electronic configuration of oxygen is
Sulfur atom uses one s-orbital and three p orbitals and thus hybridization of sulfur atom is sp3
Lewis structure of
(g)
Interpretation:
The hybrid orbitals should be predicted which is used by sulfur atom(s) in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct atoms to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
When the atomic orbitals overlap with each other in a region of high electron density, then molecular orbitals are formed. Overlapping of atomic orbitals determines the efficiency of interaction between atomic orbitals. Energy of bond molecular orbitals is less than the nonbonding molecular orbitals.
(g)
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an tom to produce two new equal orbitals. This hybridization takes place in linear molecules.
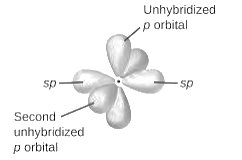
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an tom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an tom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
The given molecule is
Number of valence electrons in sulfur = 6
Number of valence electrons in fluorine = 7
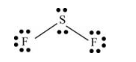
There are twofluorine atoms and one sulfur atom present in the molecule and charge on the molecule is equal to 0.
Total number of valence electrons =
Here, sulfur atom is linked with two fluorine atoms by sigma bond. By bonding in this way, each atom completes its octet.
Thus, geometry of
Electronic configuration of sulfur is
Electronic configuration of fluorine is
Sulfur atom uses one s-orbital and three p orbitals and thus hybridization of sulfur atom is sp3 implies hybrid orbitals are present.
Lewis structure of
(h)
Interpretation:
The hybrid orbitals should be predicted which is used by sulfur atom(s) in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct atoms to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
When the atomic orbitals overlap with each other in a region of high electron density, then molecular orbitals are formed. Overlapping of atomic orbitals determines the efficiency of interaction between atomic orbitals. Energy of bond molecular orbitals is less than the nonbonding molecular orbitals.
(h)
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an tom to produce two new equal orbitals. This hybridization takes place in linear molecules.
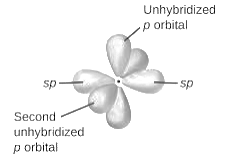
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an tom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an tom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
The given molecule is
Number of valence electrons in sulfur = 6
Number of valence electrons in fluorine = 7
There are four fluorine atoms and one sulfur atom present in the molecule and charge on the molecule is equal to 0.
Total number of valence electrons =
Here, sulfur atom is linked with four fluorine atoms by sigma bond. By bonding in this way, each atom completes its octet. Also, one lone pair of electrons is present on sulfur atom.
Thus, geometry of
Lewis structure of
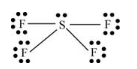
(i)
Interpretation:
The hybrid orbitals should be predicted which is used by sulfur atom(s) in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct atoms to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
When the atomic orbitals overlap with each other in a region of high electron density, then molecular orbitals are formed. Overlapping of atomic orbitals determines the efficiency of interaction between atomic orbitals. Energy of bond molecular orbitals is less than the nonbonding molecular orbitals.
(i)
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an tom to produce two new equal orbitals. This hybridization takes place in linear molecules.
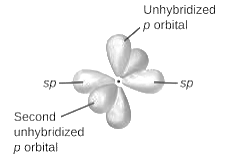
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an tom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an tom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
The given molecule is
Number of valence electrons in sulfur = 6
Number of valence electrons in fluorine = 7
There are six fluorine atoms and one sulfur atom present in the molecule and charge on the molecule is equal to 0.
Total number of valence electrons =
Here, sulfur atom is linked with six fluorine atoms by sigma bond. By bonding in this way, each atom completes its octet. No lone pair of electrons is present on sulfur atom.
Thus, geometry of
Lewis structure of
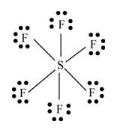
(j)
Interpretation:
The hybrid orbitals should be predicted which is used by sulfur atom(s) in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct atoms to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
When the atomic orbitals overlap with each other in a region of high electron density, then molecular orbitals are formed. Overlapping of atomic orbitals determines the efficiency of interaction between atomic orbitals. Energy of bond molecular orbitals is less than the nonbonding molecular orbitals.
(j)
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an tom to produce two new equal orbitals. This hybridization takes place in linear molecules.
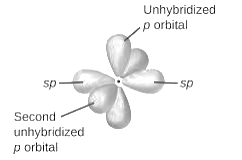
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an tom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an tom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
The given molecule is
Number of valence electrons in sulfur = 6
Number of valence electrons in fluorine = 7
There are four fluorine atoms and two sulfur atoms present in the molecule and charge on the molecule is equal to 0.
Total number of valence electrons =
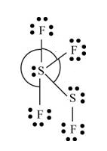
Here, two sulfur atoms are linked by sigma bond. The three fluorine atoms are linked by one sulfur atom and one fluorine atom is linked with another sulfur atom. By bonding in this way, each atom completes its octet. Also, one lone pair of electrons is present on one sulfur atom and two lone pairs of electrons are present on another sulfur atom.
Thus, geometry of
Lewis structure of
Want to see more full solutions like this?
Chapter 14 Solutions
Chemical Principles
- Hi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forwardHi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forward
- Indicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forwardDraw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forwardDraw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forward
- Draw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forwardExplain how substitutions at the 5-position of barbituric acid increase the compound's lipophilicity.arrow_forward
- Explain how substitutions at the 5-position of phenobarbital increase the compound's lipophilicity.arrow_forwardName an interesting derivative of barbituric acid, describing its structure.arrow_forwardBriefly describe the synthesis mechanism of barbituric acid from the condensation of urea with a β-diketone.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





