
(a)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(a)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
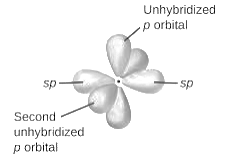
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom , present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom .
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons = 8
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 5 shows that the hybridization of central an atom is sp3d and geometry is Trigonal bipyramidal.
(b)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(b)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
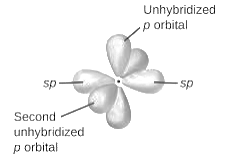
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom , present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom .
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons = 7
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 5 shows that the hybridization of central an atom is sp3d and geometry is Trigonal bipyramidal.
(c)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(c)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
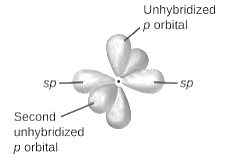
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom, present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom .
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons = 6
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 5 shows that the hybridization of central an atom is sp3d and geometry is Trigonal bipyramidal.
(d)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(d)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
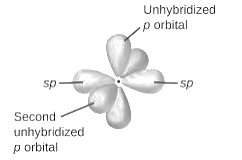
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom , present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom.
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons = 5
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 5 shows that the hybridization of central an atom is sp3d and geometry is Trigonal bipyramidal.
(a)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(a)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
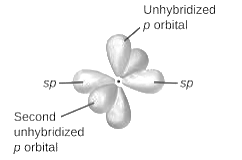
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom , present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom .
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons = 7
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 6 shows that the hybridization of central an atom is sp3d2 and geometry is octahedral.
(b)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(b)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
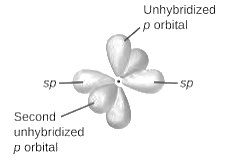
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom , present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom .
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons = 8
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 6 shows that the hybridization of central an atom is sp3d2 and geometry is octahedral.
(c)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(c)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
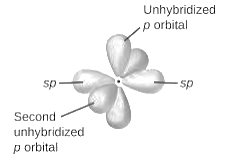
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom , present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom .
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons =6
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 6 shows that the hybridization of central an atom is sp3d2 and geometry is octahedral.
Want to see more full solutions like this?
Chapter 14 Solutions
Chemical Principles
- For which of the following ionic compounds would you expect the smallest difference between its theoretical and experimental lattice enthalpies? (You may assume these all have the same unit cell structure.) Electronegativities: Ca (1.0), Fe (1.8), Mg (1.2), O (3.5), S (2.5), Zn (1.6) Group of answer choices ZnO MgS CaO FeSarrow_forwardIn the Born-Haber cycle for KCl crystal formation, what enthalpy component must be divided by two? Group of answer choices KCl(s) enthalpy of formation Ionization energy for K(g) K(s) sublimation enthalpy Cl2 bond dissociation enthalpyarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O₂ / HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI хот Br Solvent Reagent(s) Solvent Reagent(s)arrow_forward
- What is the correct chemical equation for the lattice formation reaction for CaBr2? Group of answer choices Ca2+(g) + 2 Br−(g) → CaBr2(s) ½ Ca2+(g) + Br−(g) → ½ CaBr2(s) Ca(s) + Br2(l) → CaBr2(s) Ca(s) + 2 Br−(g) → CaBr2(s)arrow_forwardPLEASE ANSWER THE QUESTION!!!arrow_forward3. SYNTHESIS. Propose a sequence of synthetic steps (FGI) that convert the starting material (SM) into the Target molecule. For each FGI in your proposed synthesis, specify the reagents / conditions, and draw the product(s) of that FGI. DO NOT INCLUDE the FGI mxn in the answer you submit. If an FGI requires two reagent sets, specify the order in which the reagent sets are added, e.g., i) Hg(OAc)2 / H₂O; ii) NaBH4/MeOH. Indicate the stereochemistry (if any) of the products of each FGI. FGI 1. Me Starting Material Source of all carbons in the Target molecule (can use multiple copies) Me Me Target molecule + enantiomerarrow_forward
- curved arrows are used to illustate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction mechanism stepsarrow_forwardIf is was a very hot day, what would the aldol condensation product be? *see imagearrow_forwardPlease help me with number 1-3. Thank you so much.arrow_forward
- Draw the major product of this reaction ingnore the inorganic byproducts. 1. NaOCH2CH3 at 25 C 2. PhCH2Br (1 eq)arrow_forwardAt 90ºC the vapor pressure of ortho-xylene is 20 kPa and that of meta-xylene is 18 kPa. What is the composition of the vapor in equilibrium with a mixture in which the mole fraction of o-xylene is 0.60?arrow_forwardDraw the products of this reduction of a ketone with sodium borohydride. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, where applicableIgnore any inorganic byproducts. 1) NaBH4 2) HCI/H2O Select to Drawarrow_forward

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





