
Interpretation:
The given data is to be interpreted and a graph is to be drawn.
Concept introduction:
The values of radon level are noted on particular time. A line or bar graph can be formed.
Answer to Problem 11STP
A line graph is drawn showing the radon level change within the interval August 2004 to July, 2005.
Explanation of Solution
The line graph is drawn taking radon level on Y-axis and Months on X-axis.
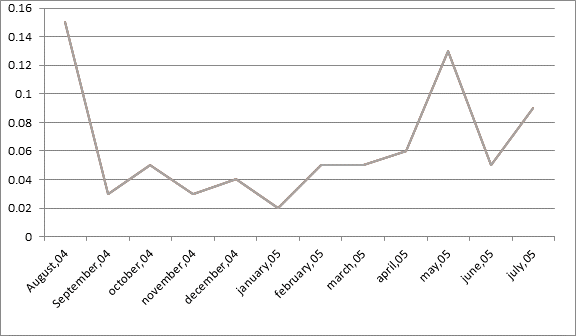
The radon level change has no particular pattern. Sometimes it increases, sometimes decreases and sometimes remain same.
Chapter 13 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Campbell Biology in Focus (2nd Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Microbiology: An Introduction
Anatomy & Physiology (6th Edition)
Applications and Investigations in Earth Science (9th Edition)
- Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds slower at temperatures below 41. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 94. °C. AS is (pick one) This reaction is always spontaneous, but ΔΗ is (pick one) C proceeds slower at temperatures below −14. °C. AS is (pick one) Х 00. 18 Ar 무ㅎ B 1 1arrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. + H CH3CH2OH HCI Drawingarrow_forwardplease explain this in simple termsarrow_forward
- K Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward(2 pts) Why is O2 more stable as a diatomic molecule than S2?arrow_forwardDraw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forward
- Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. :0: Cl C C1: 0=0: : 0 : : 0 : H C N No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. ☐ No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forwardDraw the Lewis structure for the polyatomic trisulfide anion. Be sure to include all resonance structures that satisfy the octet rule. с [ ] - Garrow_forward1. Calculate the accurate monoisotopic mass (using all 1H, 12C, 14N, 160 and 35CI) for your product using the table in your lab manual. Don't include the Cl, since you should only have [M+H]*. Compare this to the value you see on the LC-MS printout. How much different are they? 2. There are four isotopic peaks for the [M+H]* ion at m/z 240, 241, 242 and 243. For one point of extra credit, explain what each of these is and why they are present. 3. There is a fragment ion at m/z 184. For one point of extra credit, identify this fragment and confirm by calculating the accurate monoisotopic mass. 4. The UV spectrum is also at the bottom of your printout. For one point of extra credit, look up the UV spectrum of bupropion on Google Images and compare to your spectrum. Do they match? Cite your source. 5. For most of you, there will be a second chromatographic peak whose m/z is 74 (to a round number). For one point of extra credit, see if you can identify this molecule as well and confirm by…arrow_forward
- Please draw, not just describe!arrow_forwardcan you draw each step on a piece of a paper please this is very confusing to mearrow_forward> Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





