
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12.9, Problem 23P
Which of the following are
- a. hemiacetals?
- b. acetals?
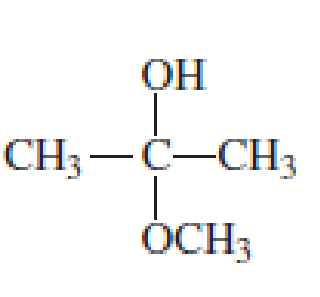
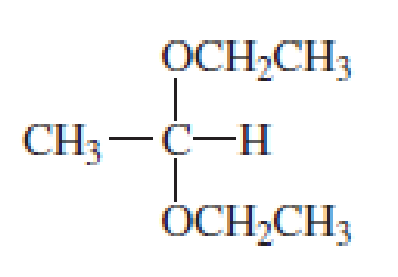
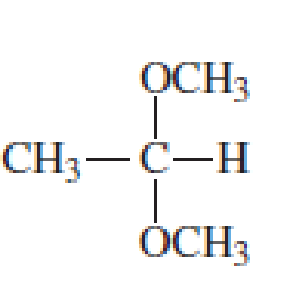
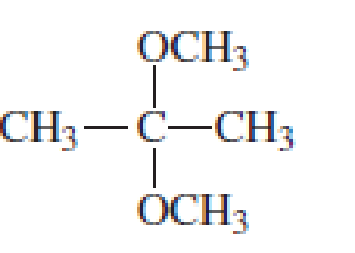
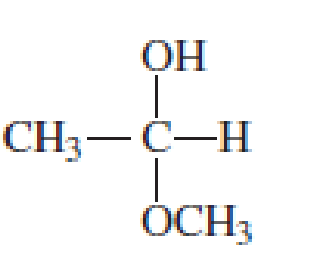
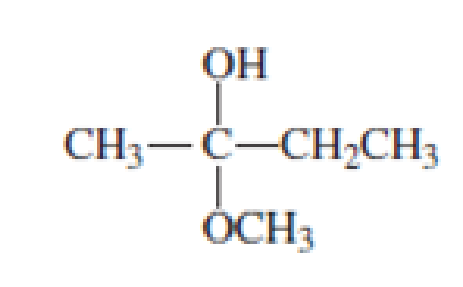
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An expression for the root mean square velocity, vrms, of a gas was derived. Using Maxwell’s velocity distribution, one can also calculate the mean velocity and the most probable velocity (mp) of a collection of molecules. The equations used for these two quantities are vmean=(8RT/πM)1/2 and vmp=(2RT/M)1/2 These values have a fixed relationship to each other.(a) Arrange these three quantities in order of increasing magnitude.(b) Show that the relative magnitudes are independent of the molar mass of the gas.(c) Use the smallest velocity as a reference for establishing the order of magnitude and determine the relationship between the larger and smaller values.
The reaction of solid dimethylhydrazine, (CH3)2N2H2, and liquefied dinitrogen tetroxide, N2O4, has been investigated for use as rocket fuel. The reaction produces the gases carbon dioxide (CO2), nitrogen (N2), and water vapor (H2O), which are ejected in the exhaust gases. In a controlled experiment, solid dimethylhydrazine was reacted with excess dinitrogen tetroxide, and the gases were collected in a closed balloon until a pressure of 2.50 atm and a temperature of 400.0 K were reached.(a) What are the partial pressures of CO2, N2, and H2O?(b) When the CO2 is removed by chemical reaction, what are the partial pressures of the remaining gases?
One liter of chlorine gas at 1 atm and 298 K reacts completely with 1.00 L of nitrogen gas and 2.00 L of oxygen gas at the same temperature and pressure. A single gaseous product is formed, which fills a 2.00 L flask at 1.00 atm and 298 K. Use this information to determine the following characteristics of the product:(a) its empirical formula;(b) its molecular formula;(c) the most favorable Lewis formula based on formal charge arguments (the central atom is N);(d) the shape of the molecule.
Chapter 12 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 12.1 - Prob. 1PCh. 12.1 - Name the following compounds:Ch. 12.1 - Prob. 3PCh. 12.1 - Prob. 4PCh. 12.2 - Prob. 5PCh. 12.4 - Draw the structure for each of the following: a....Ch. 12.4 - Prob. 7PCh. 12.5 - What products are formed when the following...Ch. 12.5 - Prob. 9PCh. 12.5 - Prob. 10P
Ch. 12.5 - Prob. 12PCh. 12.5 - Write the mechanism for the reaction of acetyl...Ch. 12.5 - Prob. 14PCh. 12.6 - Prob. 16PCh. 12.7 - Prob. 17PCh. 12.7 - Prob. 18PCh. 12.7 - Prob. 19PCh. 12.8 - Prob. 20PCh. 12.8 - Prob. 21PCh. 12.8 - Prob. 22PCh. 12.9 - Which of the following are a. hemiacetals? b....Ch. 12.9 - Prob. 24PCh. 12.9 - Prob. 25PCh. 12.10 - Prob. 26PCh. 12.11 - Prob. 27PCh. 12 - Draw the structure for each of the following: a....Ch. 12 - Prob. 29PCh. 12 - List the following compounds in order from most...Ch. 12 - Show the reagents required to form the primary...Ch. 12 - Fill in the boxes:Ch. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 34PCh. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - Prob. 38PCh. 12 - Prob. 39PCh. 12 - Show two ways the following compound could be...Ch. 12 - Prob. 41PCh. 12 - Prob. 42PCh. 12 - Prob. 43PCh. 12 - Prob. 44PCh. 12 - Prob. 45PCh. 12 - Prob. 46PCh. 12 - Prob. 47PCh. 12 - Prob. 48PCh. 12 - Prob. 49PCh. 12 - Prob. 50PCh. 12 - Prob. 51PCh. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forwardDraw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forwardBenzimidazole E. State its formula. sState the differences in the formula with other benzimidazoles.arrow_forward
- Draw product A, indicating what type of reaction occurs. F3C CN CF3 K2CO3, DMSO, H₂O2 Aarrow_forward19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forwardpls helparrow_forward
- pls helparrow_forward35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward21) The rate of reaction depends upon: a. the concentration and nature of reactants b. the temperature of the reaction C. whether or not a catalyst was used d. all of the above 22) A Maxwell-Boltzmann curve shows the distribution of molecular energies in a reaction system. When the temperature in this system is increased, the peak is a. higher and further to the right. b. higher and further to the left. c. lower and further to the right. d. lower and further to the left. 23) Which of the following correctly describes the reaction represented by the reaction below? CaCO3 (s) + energy → CaO (s) + CO2 (g) a. It is exothermic and the potential energy is greater in the reactants than the products. b. c. It is exothermic and the potential energy is greater in the products than the reactants. It is endothermic and the potential energy is greater in the products than the reactants. d. It is endothermic and the potential energy is equal for the products and reactants.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY