
(a)
Interpretation: The major product of the given reaction has to be identified.
Concept Introduction:
Major product in the reaction of
General scheme:
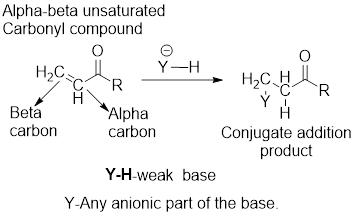
It can be observed that the conjugate addition product has been formed by the addition of basic anion to the beta-carbon atom and that of hydrogen atom to the alpha-carbon carbon atom.
The examples for the weak bases are:
(b)
Interpretation: The major product of the given reaction has to be found.
Concept Introduction:
Major product in the reaction of
General scheme:
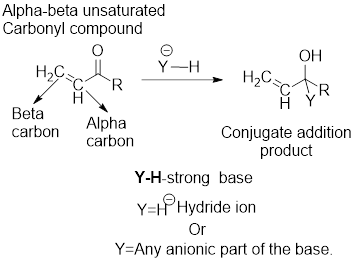
It can be observed that the direct addition product has been formed from the direct reaction of the strong base with the carbonyl
The examples for the strong bases are:
(c)
Interpretation: The major product of the given reaction has to be found.
Concept Introduction:
Major product in the reaction of
General scheme:

It can be observed that the conjugate addition product has been formed by the addition of basic anion to the beta-carbon atom and that of hydrogen atom to the alpha-carbon carbon atom.
The examples for the weak bases are:
(d)
Interpretation: The major product of the given reaction has to be found.
Concept Introduction:
Major product in the reaction of
General scheme:

It can be observed that the direct addition product has been formed from the direct reaction of the strong base with the carbonyl functional group of the
The examples for the strong bases are:
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Essential Organic Chemistry (3rd Edition)
- The quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
- If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forward
- Does Avogadro's number have units?arrow_forwardExplain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forward
- Indicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
