
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 12.5, Problem 12P
Interpretation Introduction
Interpretation:
It should be identified that the given secondary alcohols which can be prepared by the methyl formate with excess of Grignard reagent.
Concept introduction:
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as
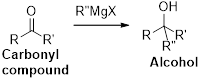
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
What is the final product when D-galactose reacts with hydroxylamine?
Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.
Chapter 12 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 12.1 - Prob. 1PCh. 12.1 - Name the following compounds:Ch. 12.1 - Prob. 3PCh. 12.1 - Prob. 4PCh. 12.2 - Prob. 5PCh. 12.4 - Draw the structure for each of the following: a....Ch. 12.4 - Prob. 7PCh. 12.5 - What products are formed when the following...Ch. 12.5 - Prob. 9PCh. 12.5 - Prob. 10P
Ch. 12.5 - Prob. 12PCh. 12.5 - Write the mechanism for the reaction of acetyl...Ch. 12.5 - Prob. 14PCh. 12.6 - Prob. 16PCh. 12.7 - Prob. 17PCh. 12.7 - Prob. 18PCh. 12.7 - Prob. 19PCh. 12.8 - Prob. 20PCh. 12.8 - Prob. 21PCh. 12.8 - Prob. 22PCh. 12.9 - Which of the following are a. hemiacetals? b....Ch. 12.9 - Prob. 24PCh. 12.9 - Prob. 25PCh. 12.10 - Prob. 26PCh. 12.11 - Prob. 27PCh. 12 - Draw the structure for each of the following: a....Ch. 12 - Prob. 29PCh. 12 - List the following compounds in order from most...Ch. 12 - Show the reagents required to form the primary...Ch. 12 - Fill in the boxes:Ch. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 34PCh. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - Prob. 38PCh. 12 - Prob. 39PCh. 12 - Show two ways the following compound could be...Ch. 12 - Prob. 41PCh. 12 - Prob. 42PCh. 12 - Prob. 43PCh. 12 - Prob. 44PCh. 12 - Prob. 45PCh. 12 - Prob. 46PCh. 12 - Prob. 47PCh. 12 - Prob. 48PCh. 12 - Prob. 49PCh. 12 - Prob. 50PCh. 12 - Prob. 51PCh. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning