
Interpretation:
Numbers are not used to designate the position of the
Concept introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry).IUPAC name consists of three parts, namely Prefix, suffix and root word.
Prefix- Represents the substituent present in the molecule and its position in the root name.
Suffix- Denotes the presence of functional group if any in the molecule. It can be an
Root word - Represents the longest continuous carbon skeleton of the organic molecule.
The functional group in the
The given compound is an aldehyde (
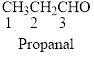
The given compound is an ketone (
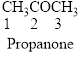
Trending nowThis is a popular solution!

Chapter 12 Solutions
Essential Organic Chemistry (3rd Edition)
- Can I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





