
Concept explainers
Interpretation:
The steps how the two products formed from reaction of methylenecyclohehane with NBS has to be given. Stereoisomers has to be disregarded.
Concept introduction:
Bromination of Allylic Carbons:
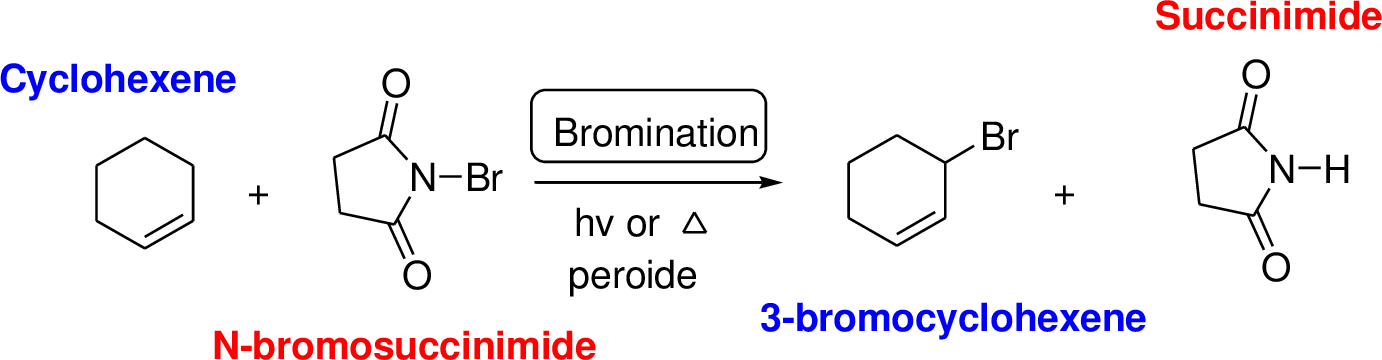
N-bromosuccinimide (NBS) is used for the allylic bromination through radical reaction. Bromination of allylicc carbon requires low concentration of bromine and low concentration of hydrobromic acid. If high concentration of bromine and high concentration of hydrobromic acid which leads to the formation of bromonation in the double bond.
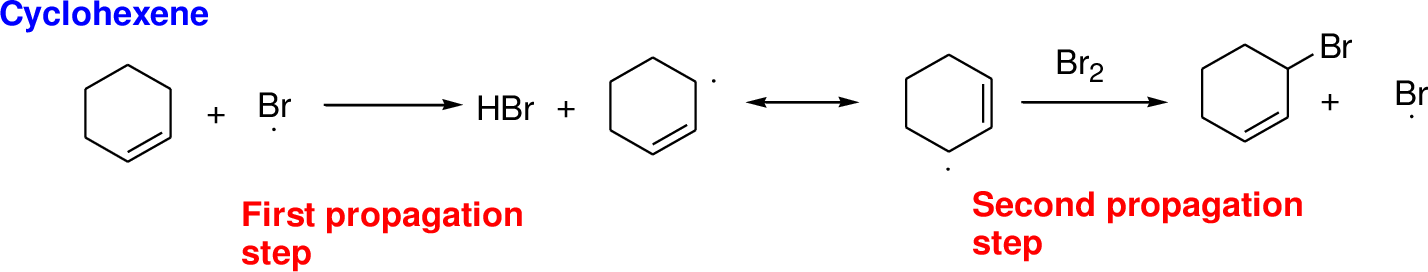
Bromination reaction starts with the homolytic cleavage of N-Br bond in N-bromosuccinimide (NBS) which creates bromine radical to initiate the radical bromination reaction.
NBS bromine radical removes the allylic hydrogen which forms hydrogen bromide and allylic radical in the first propagation step, the allylic radical is stabilized by the double bond in ring. This allylic radical reaction with bromine molecule and forms allylic bromide in the second propagation step which are shown above.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
EBK ORGANIC CHEMISTRY
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

