
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.8, Problem 16P
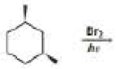
Draw the stereoisomers of the major monobromination products obtained from the following react ion.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Correctly name this compound using the IUPAC naming system by sorting the
components into the correct order.
Br
IN
Ν
H
How is the radical intermediate for this structure formed? Can you please draw arrows from the first radical to the resonance form that would result in this product? I'm lost.
Part VI.
(a) calculate the λ max of the compound using woodward - Fieser rules.
(b) what types of electronic transitions are present in the compound?
(c) what are the prominent peaks in the IR spectrum of the compound?
Chapter 12 Solutions
EBK ORGANIC CHEMISTRY
Ch. 12.2 - Prob. 1PCh. 12.2 - Write the steps for formation of...Ch. 12.3 - Prob. 3PCh. 12.4 - How many alkyl chlorides are obtained from...Ch. 12.4 - Prob. 6PCh. 12.5 - Prob. 8PCh. 12.5 - a. Would chlorination or bromination produce a...Ch. 12.5 - Show how the following compounds could be prepared...Ch. 12.6 - Prob. 12PCh. 12.7 - Prob. 13P
Ch. 12.7 - Prob. 14PCh. 12.8 - Prob. 15PCh. 12.8 - Draw the stereoisomers of the major...Ch. 12.9 - Prob. 18PCh. 12.9 - How many allylic substituted bromoalkenes are...Ch. 12.9 - a. How many stereoisomers are formed from the...Ch. 12.9 - Prob. 21PCh. 12.9 - Prob. 22PCh. 12.10 - Prob. 23PCh. 12.11 - How many atoms share the unpaired electrons in...Ch. 12.11 - Prob. 25PCh. 12 - Prob. 26PCh. 12 - Prob. 27PCh. 12 - Prob. 28PCh. 12 - Prob. 29PCh. 12 - Prob. 30PCh. 12 - Prob. 31PCh. 12 - Prob. 32PCh. 12 - Prob. 33PCh. 12 - Prob. 34PCh. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - a. What five-carbon alkene forms the same product...Ch. 12 - Prob. 39PCh. 12 - Starting with cyclohexane, how could the following...Ch. 12 - a. Propose a mechanism for the following reaction:...Ch. 12 - What stereoisomers are obtained from the following...Ch. 12 - Prob. 43PCh. 12 - Prob. 44PCh. 12 - Prob. 45PCh. 12 - Draw the products of the following reactions,...Ch. 12 - Prob. 47PCh. 12 - Prob. 48PCh. 12 - Prob. 49PCh. 12 - Explain why the rate of bromination of methane...Ch. 12 - Prob. 51PCh. 12 - Prob. 1PCh. 12 - Prob. 2PCh. 12 - Prob. 3PCh. 12 - Prob. 4P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forward
- Briefly indicate the structure and bonding of silicates.arrow_forward4 Part C Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma.arrow_forwardTry: Draw possible resonance contributing structures for the following organic species: CH3CH2NO2 [CH2CHCH2] [CH2CHCHO] [CH2CHCH2] [CH2CHNH2]arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License