
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.4, Problem 4P
How many alkyl chlorides are obtained from monochlorination of the following

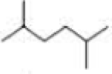
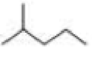

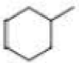
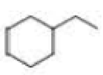
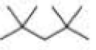

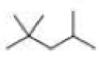
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the normal hydrogen electrode, the balance potential difference in the interface is 0 mV, the maximum potential is 5 mV. Explain briefly.
utron
eutro
cle
TH
tro
(Na
(b) Atoms are said to be electrically neutral. Explain.
(c)
Distinguish
between the following:
(i) Atomic number and mass number.
(ii) Mass number and relative atomic mass.
2. An isotope Q, has 18 neutrons a mass number of 34.
(a) (i) Draw the atomic structure of Q.
(ii) Write its electron arrangement
(b) To which period and group does Q belong? Explain your answer.
(c) How does Q form its ion? Explain.
3. (a) Determine the relative atomic mass of the following elements =
compositions occur in the proportions given.
(i) Neon
20
21
22.
Ne (90.92%), 10Ne (0.26%), and 10Ne (8.82%)
(ii) Argon
36
38
40
18 Ar (0.34%), 18 Ar (0.06%) and 18 Ar (99.6%)
In the normal hydrogen electrode, the balance potential difference in the interface is this, the maximum potential is 5 mV. Explain briefly.
Chapter 12 Solutions
EBK ORGANIC CHEMISTRY
Ch. 12.2 - Prob. 1PCh. 12.2 - Write the steps for formation of...Ch. 12.3 - Prob. 3PCh. 12.4 - How many alkyl chlorides are obtained from...Ch. 12.4 - Prob. 6PCh. 12.5 - Prob. 8PCh. 12.5 - a. Would chlorination or bromination produce a...Ch. 12.5 - Show how the following compounds could be prepared...Ch. 12.6 - Prob. 12PCh. 12.7 - Prob. 13P
Ch. 12.7 - Prob. 14PCh. 12.8 - Prob. 15PCh. 12.8 - Draw the stereoisomers of the major...Ch. 12.9 - Prob. 18PCh. 12.9 - How many allylic substituted bromoalkenes are...Ch. 12.9 - a. How many stereoisomers are formed from the...Ch. 12.9 - Prob. 21PCh. 12.9 - Prob. 22PCh. 12.10 - Prob. 23PCh. 12.11 - How many atoms share the unpaired electrons in...Ch. 12.11 - Prob. 25PCh. 12 - Prob. 26PCh. 12 - Prob. 27PCh. 12 - Prob. 28PCh. 12 - Prob. 29PCh. 12 - Prob. 30PCh. 12 - Prob. 31PCh. 12 - Prob. 32PCh. 12 - Prob. 33PCh. 12 - Prob. 34PCh. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - a. What five-carbon alkene forms the same product...Ch. 12 - Prob. 39PCh. 12 - Starting with cyclohexane, how could the following...Ch. 12 - a. Propose a mechanism for the following reaction:...Ch. 12 - What stereoisomers are obtained from the following...Ch. 12 - Prob. 43PCh. 12 - Prob. 44PCh. 12 - Prob. 45PCh. 12 - Draw the products of the following reactions,...Ch. 12 - Prob. 47PCh. 12 - Prob. 48PCh. 12 - Prob. 49PCh. 12 - Explain why the rate of bromination of methane...Ch. 12 - Prob. 51PCh. 12 - Prob. 1PCh. 12 - Prob. 2PCh. 12 - Prob. 3PCh. 12 - Prob. 4P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The electrode balance potential is -0.118 V and the interface potential difference is +5 mV. The overvoltage n will be 0.005 - (-0.118) = 0.123 V. Is it correct?arrow_forwardIn the electrode Pt, H2(1 atm) | H+(a=1), if the electrode balance potential is -0.118 V and the interface potential difference is +5 mV. The current voltage will be 0.005 - (-0.118) = 0.123 V ¿Correcto?arrow_forwardIn the electrode Pt, H2(1 atm) | H+(a=1) at 298K is 0.79 mA cm-2. If the balance potential of the electrode is -0.118 V and the potential difference of the interface is +5 mV. Determine its potential.arrow_forward
- In one electrode: Pt, H2(1 atm) | H+(a=1), the interchange current density at 298K is 0.79 mA·cm-2. If the voltage difference of the interface is +5 mV. What will be the correct intensity at pH = 2?. Maximum transfer voltage and beta = 0.5.arrow_forwardIn a Pt electrode, H2(1 atm) | H+(a=1), the interchange current density of an electrode is 0.79 mA cm-2. ¿Qué corriente flow across the electrode of área 5 cm2 when the difference in potential of the interface is +5 mV?.arrow_forwardIf the current voltage is n = 0.14 V, indicate which of the 2 voltage formulas of the ley of Tafel must be applied i a a) == exp (1-B). xp[(1 - ß³): Fn Fn a b) == exp B RT RTarrow_forward
- If the current voltage is n = 0.14 V. Indicate which of the 2 formulas must be applied a) = a T = i exp[(1 - p) F Fn Fn b) i==exp B RTarrow_forwardTopic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTwo cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 Carrow_forward
- With the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.arrow_forwardApply the NANSTE law to the MnO4- + 8H+ + 5e- ⇄ Mn2+ + 4H2Oarrow_forwardIn the Nernst Law, how much is RT / F?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY