
(a)
Interpretation:
It should be determined that the amide used to produce Benzylmethylamine on reaction with
Concept introduction:
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent.
Lithium aluminium hydride is reduced the amide as an
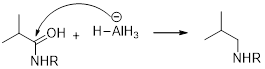
(b)
Interpretation:
It should be determined that the amide used to produce Ethylamine on rection with
Concept introduction:
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent.
Lithium aluminium hydride is reduced the amide as an amine which is shown below.
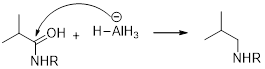
(c)
Interpretation:
It should be determined that the amide used to produce Diethylamine on rection with
Concept introduction:
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent.
Lithium aluminium hydride is reduced the amide as an amine which is shown below.
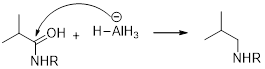
(d)
Interpretation:
It should be determined that the amide used to produce Triethylamine on rection with
Concept introduction:
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent.
Lithium aluminium hydride is reduced the amide as an amine which is shown below.
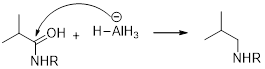
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
- What is the pH of the Tris buffer after the addition of 10 mL of 0.01M NaOH? How would I calculate this?arrow_forwardWhy do isopolianions form polymeric species with a defined molecular weight? What does it depend on?arrow_forwardWhat are isopolianions? Describe the structural unit of isopolianions.arrow_forward
- Justify the polymerization of vanadates VO43-, as a function of concentration and pH.arrow_forwardWhat is the preparation of 500 mL of 100mM MOPS buffer (pH=7.5) starting with 1 M MOPS and 1 M NaOH? How would I calculate the math?arrow_forwardIndicate the correct option.a) Isopolianions are formed around metallic atoms in a low oxidation state.b) Non-metals such as N, S, C, Cl, ... give rise to polyacids (oxygenated).c) Both are incorrect.arrow_forward
- 14. Which one of the compounds below is the major organic product obtained from the following series of reactions? Br OH OH CH3O™ Na+ H*, H₂O SN2 HO OH A B C D 0 Earrow_forwardWavelength (nm) I'm not sure what equation I can come up with other than the one generated with my graph. Can you please show me the calculations that were used to find this equation? Give an equation that relates energy to wavelength. Explain how you arrived at your equation. Wavelength Energy (kJ/mol) (nm) 350 341.8 420 284.8 470 254.5 530 225.7 580 206.3 620 192.9 700 170.9 750 159.5 Energy vs. Wavelength (Graph 1) 400 350 y=-0.4367x+470.82 300 250 200 150 100 50 O 0 100 200 300 400 500 600 700 800 Energy (kJ/mol)arrow_forward5. Draw molecular orbital diagrams for superoxide (O2¯), and peroxide (O2²-). A good starting point would be MO diagram for O2 given in your textbook. Then: a) calculate bond orders in superoxide and in peroxide; indicate which species would have a stronger oxygen-oxygen bond; b) indicate which species would be a radical. (4 points)arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





