
Concept explainers
(a)
Interpretation:
It should be determined that the product obtained from the reaction of Ethyl butanoate with
Concept introduction:
Reduction of carbonyl, carboxylic acid and ester using
Reduction of an ester (Methyl-2-pentenoate) by using
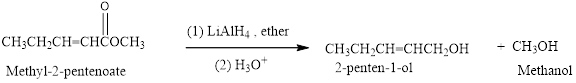
(b)
Interpretation:
It should be determined that the product obtained from the reaction of Methyl benzoate with
Concept introduction:
Reduction of carbonyl, carboxylic acid and ester using
Reduction of an ester (Methyl-2-pentenoate) by using
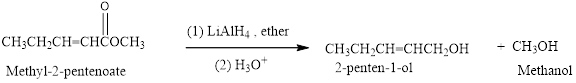
(c)
Interpretation:
It should be determined that the product obtained from the reaction of Pentatonic acid
with
Concept introduction:
Reduction of carbonyl, carboxylic acid and ester using
Reduction of a carboxylic acid (oleic acid) by using
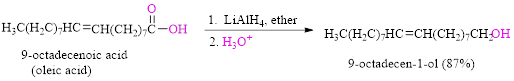
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
- Don't used hand raitingarrow_forwardShown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. H. +N=C H H H Cl: Click and drag to start drawing a structure. : ? g B S olo Ar B Karrow_forwardDon't used hand raitingarrow_forward
- S Shown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H H = HIN: H C. :0 H /\ H H Click and drag to start drawing a structure. ×arrow_forwardPlease help me figure out these calculation and what should be plotted. These are notes for my chemistry class.arrow_forwardNonearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole





