
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
3rd Edition
ISBN: 9780137533268
Author: Paula Bruice
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 12, Problem 49P
Interpretation Introduction
Interpretation:
The mechanism for the given reaction has to be determined.
Concept introduction:
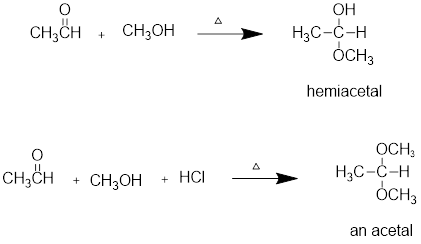
Addition of alcohols to
Aldehyde reacts with alcohol to form Hemiacetals or acetals. For example, the reaction of methanol with ethanol to form Hemiacetals or acetals.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting
Don't used hand raiting
Don't used hand raiting
Chapter 12 Solutions
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
Ch. 12.1 - Prob. 1PCh. 12.1 - Name the following compounds:Ch. 12.1 - Prob. 3PCh. 12.1 - Prob. 4PCh. 12.2 - Prob. 5PCh. 12.4 - Draw the structure for each of the following: a....Ch. 12.4 - Prob. 7PCh. 12.5 - What products are formed when the following...Ch. 12.5 - Prob. 9PCh. 12.5 - Prob. 10P
Ch. 12.5 - Prob. 12PCh. 12.5 - Write the mechanism for the reaction of acetyl...Ch. 12.5 - Prob. 14PCh. 12.6 - Prob. 16PCh. 12.7 - Prob. 17PCh. 12.7 - Prob. 18PCh. 12.7 - Prob. 19PCh. 12.8 - Prob. 20PCh. 12.8 - Prob. 21PCh. 12.8 - Prob. 22PCh. 12.9 - Which of the following are a. hemiacetals? b....Ch. 12.9 - Prob. 24PCh. 12.9 - Prob. 25PCh. 12.10 - Prob. 26PCh. 12.11 - Prob. 27PCh. 12 - Draw the structure for each of the following: a....Ch. 12 - Prob. 29PCh. 12 - List the following compounds in order from most...Ch. 12 - Show the reagents required to form the primary...Ch. 12 - Fill in the boxes:Ch. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 34PCh. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - Prob. 38PCh. 12 - Prob. 39PCh. 12 - Show two ways the following compound could be...Ch. 12 - Prob. 41PCh. 12 - Prob. 42PCh. 12 - Prob. 43PCh. 12 - Prob. 44PCh. 12 - Prob. 45PCh. 12 - Prob. 46PCh. 12 - Prob. 47PCh. 12 - Prob. 48PCh. 12 - Prob. 49PCh. 12 - Prob. 50PCh. 12 - Prob. 51PCh. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A DEPT NMR spectrum is shown for a molecule with the molecular formula of C5H12O. Draw the structure that best fits this data. 200 180 160 140 120 100 一盆 00 40 8- 20 ppm 0 Qarrow_forwardDon't used hand raitingarrow_forwardShown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. H. +N=C H H H Cl: Click and drag to start drawing a structure. : ? g B S olo Ar B Karrow_forward
- Don't used hand raitingarrow_forwardS Shown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H H = HIN: H C. :0 H /\ H H Click and drag to start drawing a structure. ×arrow_forwardPlease help me figure out these calculation and what should be plotted. These are notes for my chemistry class.arrow_forward
- Nonearrow_forwardNonearrow_forwardPart II. two unbranched ketone have molecular formulla (C8H100). El-ms showed that both of them have a molecular ion peak at m/2 =128. However ketone (A) has a fragment peak at m/2 = 99 and 72 while ketone (B) snowed a fragment peak at m/2 = 113 and 58. 9) Propose the most plausible structures for both ketones b) Explain how you arrived at your conclusion by drawing the Structures of the distinguishing fragments for each ketone, including their fragmentation mechanisms.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License