
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
3rd Edition
ISBN: 9780137533268
Author: Paula Bruice
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Question
Chapter 12.5, Problem 9P
Interpretation Introduction
Interpretation:
The other combination of
Concept introduction:
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as
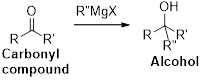
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the preparation of 500 mL of 100mM MOPS buffer (pH=7.5) starting with 1 M MOPS and 1 M NaOH? How would I calculate the math?
Indicate the correct option.a) Isopolianions are formed around metallic atoms in a low oxidation state.b) Non-metals such as N, S, C, Cl, ... give rise to polyacids (oxygenated).c) Both are incorrect.
14. Which one of the compounds
below is the major organic
product obtained from the
following series of reactions?
Br
OH
OH
CH3O™ Na+
H*, H₂O
SN2
HO
OH
A
B
C
D
0
E
Chapter 12 Solutions
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
Ch. 12.1 - Prob. 1PCh. 12.1 - Name the following compounds:Ch. 12.1 - Prob. 3PCh. 12.1 - Prob. 4PCh. 12.2 - Prob. 5PCh. 12.4 - Draw the structure for each of the following: a....Ch. 12.4 - Prob. 7PCh. 12.5 - What products are formed when the following...Ch. 12.5 - Prob. 9PCh. 12.5 - Prob. 10P
Ch. 12.5 - Prob. 12PCh. 12.5 - Write the mechanism for the reaction of acetyl...Ch. 12.5 - Prob. 14PCh. 12.6 - Prob. 16PCh. 12.7 - Prob. 17PCh. 12.7 - Prob. 18PCh. 12.7 - Prob. 19PCh. 12.8 - Prob. 20PCh. 12.8 - Prob. 21PCh. 12.8 - Prob. 22PCh. 12.9 - Which of the following are a. hemiacetals? b....Ch. 12.9 - Prob. 24PCh. 12.9 - Prob. 25PCh. 12.10 - Prob. 26PCh. 12.11 - Prob. 27PCh. 12 - Draw the structure for each of the following: a....Ch. 12 - Prob. 29PCh. 12 - List the following compounds in order from most...Ch. 12 - Show the reagents required to form the primary...Ch. 12 - Fill in the boxes:Ch. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 34PCh. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - Prob. 38PCh. 12 - Prob. 39PCh. 12 - Show two ways the following compound could be...Ch. 12 - Prob. 41PCh. 12 - Prob. 42PCh. 12 - Prob. 43PCh. 12 - Prob. 44PCh. 12 - Prob. 45PCh. 12 - Prob. 46PCh. 12 - Prob. 47PCh. 12 - Prob. 48PCh. 12 - Prob. 49PCh. 12 - Prob. 50PCh. 12 - Prob. 51PCh. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Wavelength (nm) I'm not sure what equation I can come up with other than the one generated with my graph. Can you please show me the calculations that were used to find this equation? Give an equation that relates energy to wavelength. Explain how you arrived at your equation. Wavelength Energy (kJ/mol) (nm) 350 341.8 420 284.8 470 254.5 530 225.7 580 206.3 620 192.9 700 170.9 750 159.5 Energy vs. Wavelength (Graph 1) 400 350 y=-0.4367x+470.82 300 250 200 150 100 50 O 0 100 200 300 400 500 600 700 800 Energy (kJ/mol)arrow_forward5. Draw molecular orbital diagrams for superoxide (O2¯), and peroxide (O2²-). A good starting point would be MO diagram for O2 given in your textbook. Then: a) calculate bond orders in superoxide and in peroxide; indicate which species would have a stronger oxygen-oxygen bond; b) indicate which species would be a radical. (4 points)arrow_forward16. Which one of the compunds below is the final product of the reaction sequence shown here? عملاء .OH Br. (CH3)2CH-C=C H+,H,O 2 mol H2, Pt A OH B OH D OH E OH C OHarrow_forward
- Indicate whether any of the two options is correct.a) The most common coordination structure for isopolianions is the prismb) Heteropolianions incorporate alkaline cations into their structuresarrow_forwardPlease correct answer and don't use hand ratingarrow_forwardWavelength (nm) I'm not sure what equation I can come up with other than the one generated with my graph. Can you please show me the calculations that were used to find this equation? Give an equation that relates energy to wavelength. Explain how you arrived at your equation. Wavelength Energy (kJ/mol) (nm) 350 341.8 420 284.8 470 254.5 530 225.7 580 206.3 620 192.9 700 170.9 750 159.5 Energy vs. Wavelength (Graph 1) 400 350 y=-0.4367x+470.82 300 250 200 150 100 50 O 0 100 200 300 400 500 600 700 800 Energy (kJ/mol)arrow_forward
- 6. For the following molecules: draw Lewis dot-structures; use VSEPR method to determine geometries of the following molecules/ions. Are the central atoms in these molecules/ions considered of normal valency, or are they hypervalent? (please read paragraph 2.6) a) BrF3 (6 points) b) BrF4 c) IF₂ 4arrow_forwardNonearrow_forward7. Use Pauling's electronegativity values (Table 1.7) and Ketelaar triangle (Fig. 2.28) to classify bonding in: (3 points) a) CIF3 b) ZnCl2 c) PbSarrow_forward
- 7. What is the IUPAC name of the following compound? A) (R)-1-oxo-2-butanol C) (R)-2-hydroxybutanal E) (S)-1-formyl-1-propanol B) (S)-1-oxo-2-butanol D) (S)-2-hydroxybutanal OH Harrow_forwardCual es la formula semidesarrollada del 3-metil-1-butino?arrow_forward2. A graph shown below shows first ionization energies for elements from H to Ne. First ionization energy/kJ mol 2500 2000 1500 1000 500 T T T T 1 2 3 5 6 7 8 9 10 Atomic number a) Using arguments of electronic structure, explain why ionization energy of Li is much lower than that of H. (2 points) then dips at O. b) Using the same arguments, explain why ionization energy increases from B to N, and (3 points)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
Seven Name Reactions in One - Palladium Catalysed Reaction (047 - 053); Author: Rasayan Academy - Jagriti Sharma;https://www.youtube.com/watch?v=5HEKTpDFkqI;License: Standard YouTube License, CC-BY