
(a)
Interpretation: The products for the given reaction have to be identified.
Concept Introduction:
Ketals:
Ketals are used to protect the carbonyl group of the
In the following example, the carbonyl group of the acetone has been protected as ketal by using ethylene glycol.
Example:
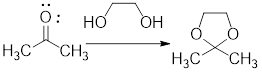
In acidic condition, a ketone reacts with two molecules of alcohol or a molecule of
General scheme:
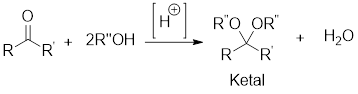
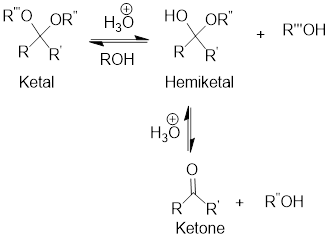
Hydrolysis of Ketal:
Hydrolysis of ketal gives back the corresponding alcohol and the corresponding ketone from which the ketal has been formed. The hydrolysis takes place only in acidic conditions. So, the hydrolysis of ketal is an acid-catalyzed hydrolysis reaction.
General scheme:
(b)
Interpretation: The products for the given reaction have to be found.
Concept Introduction:
Ketals:
Ketals are used to protect the carbonyl group of the ketone.
In the following example, the carbonyl group of the acetone has been protected as ketal by using ethylene glycol.
Example:

In acidic condition, a ketone reacts with two molecules of alcohol or a molecule of diol to form ketal or cyclic ketal respectively.
General scheme:
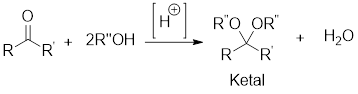
Hydrolysis of Ketal:
Hydrolysis of ketal gives back the corresponding alcohol and the corresponding ketone from which the ketal has been formed. The hydrolysis takes place only in acidic conditions. So, the hydrolysis of ketal is an acid-catalyzed hydrolysis reaction.
General scheme:

Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
- In the phase diagram of steel (two components Fe and C), region A is the gamma austenite solid and region B contains the gamma solid and liquid. Indicate the degrees of freedom that the fields A and B have,arrow_forwardFor a condensed binary system in equilibrium at constant pressure, indicate the maximum number of phases that can exist.arrow_forwardPart V. Label ad match the carbons in compounds Jane and Diane w/ the corresponding peak no. in the Spectra (Note: use the given peak no. To label the carbons, other peak no are intentionally omitted) 7 4 2 -0.13 -0.12 -0.11 -0.10 -0.08 8 CI Jane 1 -0.09 5 210 200 190 180 170 160 150 140 130 120 110 100 -8 90 f1 (ppm) 11 8 172.4 172.0 f1 (ppr HO CI NH Diane 7 3 11 80 80 -80 -R 70 60 60 2 5 -8 50 40 8. 170 160 150 140 130 120 110 100 90 -0 80 70 20 f1 (ppm) 15 30 -20 20 -60 60 -0.07 -0.06 -0.05 -0.04 -0.03 -0.02 -0.01 -0.00 -0.01 10 -0.17 16 15 56 16 -0.16 -0.15 -0.14 -0.13 -0.12 -0.11 -0.10 -0.09 -0.08 -0.07 -0.06 -0.05 -0.04 17.8 17.6 17.4 17.2 17.0 f1 (ppm) -0.03 -0.02 550 106 40 30 20 20 -0.01 -0.00 F-0.01 10 0arrow_forward
- n Feb 3 A T + 4. (2 pts) Draw the structure of the major component of the Limonene isolated. Explain how you confirmed the structure. 5. (2 pts) Draw the fragment corresponding to the base peak in the Mass spectrum of Limonene. 6. (1 pts) Predict the 1H NMR spectral data of R-Limonene. Proton NMR: 5.3 pon multiplet (H Ringarrow_forwardPart VI. Ca H 10 O is the molecular formula of compound Tom and gives the in the table below. Give a possible structure for compound Tom. 13C Signals summarized C1 C2 C3 C4 C5 C6 C7 13C shift (ppm) 23.5 27.0 33.0 35.8 127 162 205 DEPT-90 + DEPT-135 + +arrow_forward2. Using the following data to calculate the value of AvapH o of water at 298K. AvapH o of water at 373K is 40.7 kJ/mol; molar heat capacity of liquid water at constant pressure is 75.2J mol-1 K-1 and molar heat capacity of water vapor at constant pressure is 33.6 J mol-1 K-1.arrow_forward
- Part VII. Below are the 'HNMR 13 3 C-NMR, COSY 2D- NMR, and HSQC 20-NMR (Similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H13 O. Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum ли 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 f1 (ppm)arrow_forward3. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-pentene. expanded structure: Condensed structure: Skeletal formula: 4. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-methyl-3-heptene. expanded structure: Condensed structure: Skeletal formula: following structurearrow_forwardPart IV. Propose a plausible Structure w/ the following descriptions: a) A 5-carbon hydrocarbon w/ a single peak in its proton decoupled the DEPT-135 Spectrum shows a negative peak C-NMR spectrum where b) what cyclohexane dione isomer gives the largest no. Of 13C NMR signals? c) C5H120 (5-carbon alcohol) w/ most deshielded carbon absent in any of its DEPT Spectivaarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





