
An adiabatic 0.2-m3 storage tank that is initially evacuated is connected to a supply line that carries nitrogen at 225 K and 10 MPa. A valve is opened, and nitrogen flows into the tank from the supply line. The valve is closed when the pressure in the tank reaches 10 MPa. Determine the final temperature in the tank (a) treating nitrogen as an ideal gas, and (b) using generalized charts. Compare your results to the actual value of 293 K.
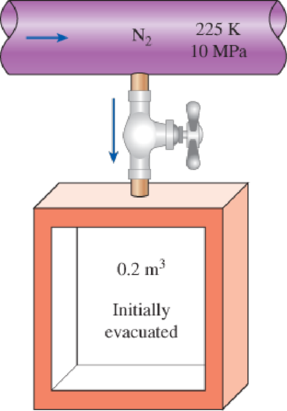
FIGURE P12–101
(a)
The final temperature in the tank by treating nitrogen as an ideal gas and compare the result to the actual value of
Answer to Problem 92RP
The final temperature in the tank by treating nitrogen as an ideal gas is
Explanation of Solution
Write the equation of mass balance.
Here, the inlet mass is
The change in mass of the system for the control volume is expressed as,
Here, the suffixes 1 and 2 indicates the initial and final states of the system.
Consider the given insulated tank as the control volume.
The valve is closed when the pressure in tank reaches to
Rewrite the Equation (I) as follows.
Write the energy balance equation.
Here, the heat transfer is
Since the tank is adiabatic, there is no heat transfer i.e.
The Equation (III) reduced as follows.
Substitute
Express the Equation (V) in molar basis.
Here, the molar mass of nitrogen is
Conclusion:
The inlet condition of the nitrogen is
While considering the nitrogen as the ideal gas, its enthalpy is solely depends on temperature.
Refer Table A-18, “Ideal-gas properties of nitrogen,
The molar enthalpy of nitrogen corresponding to the temperature of
Refer Equation (VI).
The final temperature of the nitrogen is expressed as follows.
Refer Table A-18, “Ideal-gas properties of nitrogen,
The final temperature
Thus, the final temperature in the tank by treating nitrogen as an ideal gas is
The percentage error with the actual temperature value of
The error associated is
(b)
The final temperature in the tank by using generalized departure charts.
Answer to Problem 92RP
The final temperature in the tank by using generalized departure charts is
Explanation of Solution
Refer Table A-1, “Molar mass, gas constant, and critical-point properties”.
The critical temperature and pressure of nitrogen gas is as follows.
The reduced pressure
At inlet:
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Write formula for enthalpy departure factor
Here, the inlet molar enthalpy at ideal gas state is
Rearrange the Equation (I) to obtain
Write the formula for molar enthalpy at final state
Write the formula for molar internal energy at final state.
Here, the compressibility factor is
The universal gas constant
Conclusion:
Refer part (a) answer for
Substitute
Refer Equation (VI).
It is given that the actual final temperature of nitrogen is
Consider the exit temperature
The reduced pressure
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Refer Figure A-15, “Nelson–Obert generalized compressibility chart”.
The compressibility factor
Refer Table A-18, “Ideal-gas properties of nitrogen,
The final molar enthalpy of nitrogen
Substitute
Substitute
Consider the exit temperature
The reduced pressure
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Refer Figure A-15, “Nelson–Obert generalized compressibility chart”.
The compressibility factor
Refer Table A-18, “Ideal-gas properties of nitrogen,
The final molar enthalpy of nitrogen
Substitute
Substitute
Express interpolation formula to determine the final temperature
Substitute
Thus, the final temperature in the tank by using generalized departure charts is
The percentage error with the actual temperature value of
The error associated is
Want to see more full solutions like this?
Chapter 12 Solutions
Thermodynamics: An Engineering Approach
- I need solutionsarrow_forward3-137arrow_forwardLarge wind turbines with a power capacity of 8 MW and blade span diameters of over 160 m areavailable for electric power generation. Consider a wind turbine with a blade span diameter of 120m installed at a site subjected to steady winds at 8.25 m/s. Taking the overall efficiency of thewind turbine to be 33 percent and the air density to be 1.25 kg/m3, determine the electric powergenerated by this wind turbine. Also, assuming steady winds of 8.25 m/s during a 24-h period,determine the amount of electric energy and the revenue generated per day for a unit price of$0.08/kWh for electricity.arrow_forward
- The basic barometer can be used to measure the height of a building. If the barometric readingsat the top and at the bottom of a building are 672 and 696 mmHg, respectively, determine theheight of the building. Take the densities of air and mercury to be 1.18 kg/m3 and 13,600 kg/m3,respectivelyarrow_forwardA 7.25-hp (shaft) pump is used to raise water to an elevation of 17 m. If the mechanical efficiencyof the pump is 84 percent, determine the maximum volume flow rate of water.arrow_forwardConsider a double-fluid manometer attached to an air pipe shown below. If the specific gravity ofone fluid is 13.8, determine the specific gravity of the other fluid for the indicated absolutepressure of air. Take the atmospheric pressure to be 95 kPaarrow_forward
- A race car enters the circular portion of a track that has a radius of 65 m. Disregard the 70 m in the picture. When the car enters the curve at point P, it is traveling with a speed of 120 km/h that is increasing at 5 m/s^2 . Three seconds later, determine the x and y components of velocity and acceleration of the car. I'm having trouble getting the correct y component of acceleration. all the other answers are correct. thank you!arrow_forwardFigure: 06_P041 Copyright 2013 Pearson Education, publishing a Prentice Hall 2. Determine the force that the jaws J of the metal cutters exert on the smooth cable C if 100-N forces are applied to the handles. The jaws are pinned at E and A, and D and B. There is also a pin at F. 400 mm 15° 20 mm A 15° 15 D B 30 mm² 80 mm 20 mm 400 mm Figure: 06_P090 Copyright 2013 Pearson Education, publishing as Prentice Hall 15° 100 N 100 N 15°arrow_forwardA telemetry system is used to quantify kinematic values of a ski jumper immediately before the jumper leaves the ramp. According to the system r=560 ft , r˙=−105 ft/s , r¨=−10 ft/s2 , θ=25° , θ˙=0.07 rad/s , θ¨=0.06 rad/s2 Determine the velocity of the skier immediately before leaving the jump. The velocity of the skier immediately before leaving the jump along with its direction is ? I have 112.08 ft/s but can't seem to get the direction correct. Determine the acceleration of the skier at this instant. At this instant, the acceleration of the skier along with its direction is ? acceleration is 22.8 ft/s^2 but need help with direction. Need help with velocity direction and acceleration direction please.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





