
Concept explainers
Explain why each name is incorrect and then write a correct name. Strategy: First draw the structural formula suggested by the incorrect name. Then identify the error, for example, failure to locate the longest chain that contains the
(a) 1-Methylpropene (b) 3-Pentene
(c) 2-Methylcyclohexene (d) 3,3-Dimethylpentene
(e) 4-Hexyne
(f) 2-lsopropyl-2-butene
(a)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
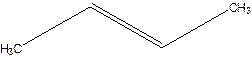
But-2-ene.
Explanation of Solution
1-Methylpropene.
Error- longest chain not correctly located.
Correct name-
But-2-ene.
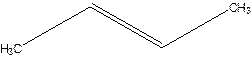
But-2-ene.
(b)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
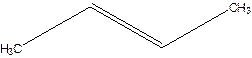
Answer to Problem 16P
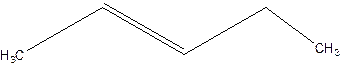
2-pentene.
Explanation of Solution
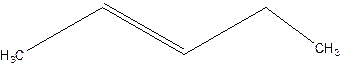
3-Pentene.
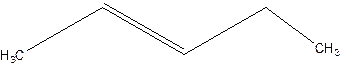
Error- Position of double bond not mentioned correctly.
Correct name-
2-Pentene.
(c)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
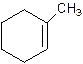
1-Methylcyclo-1-hexene.
Explanation of Solution
2-Methylcyclohexene.
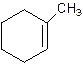
Error- Position of methyl not correct.
Correct name-
1-Methylcyclo-1-hexene.
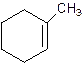
(d)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
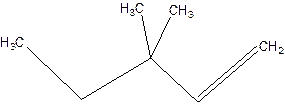
3,3-Dimethyl-1-pentene.
Explanation of Solution
3,3-Dimethylpentene.
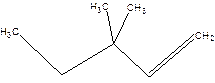
Error-position of double bond not mentioned.
Correct name-
3,3-Dimethyl-1-pentene.
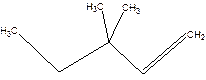
(e)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
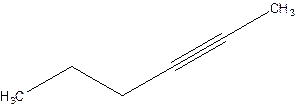
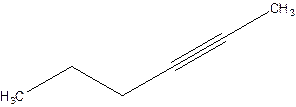
2-Hexyne.
Explanation of Solution
4-Hexyne.
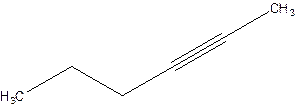
Error- position of triple bond is incorrectly mentioned.
Correct name 2-Hexyne.
(f)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
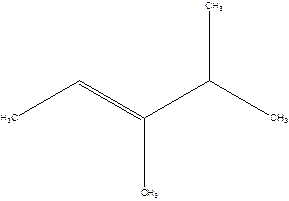
3,4-Dimethyl-2-pentene.
Explanation of Solution
2-Isopropyl-2-butene.
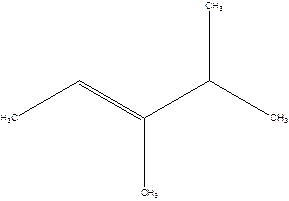
Error- longest chain is not correctly located.
The correct name will be-
3,4-Dimethyl-2-pentene.
Want to see more full solutions like this?
Chapter 12 Solutions
Introduction To General, Organic, And Biochemistry
- Part VII. Below are the 'HNMR 13 3 C-NMR, COSY 2D- NMR, and HSQC 20-NMR (Similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H13 O. Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum ли 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 f1 (ppm)arrow_forward3. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-pentene. expanded structure: Condensed structure: Skeletal formula: 4. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-methyl-3-heptene. expanded structure: Condensed structure: Skeletal formula: following structurearrow_forwardPart IV. Propose a plausible Structure w/ the following descriptions: a) A 5-carbon hydrocarbon w/ a single peak in its proton decoupled the DEPT-135 Spectrum shows a negative peak C-NMR spectrum where b) what cyclohexane dione isomer gives the largest no. Of 13C NMR signals? c) C5H120 (5-carbon alcohol) w/ most deshielded carbon absent in any of its DEPT Spectivaarrow_forward
- 13C NMR is good for: a) determining the molecular weight of the compound b) identifying certain functional groups. c) determining the carbon skeleton, for example methyl vs ethyl vs propyl groups d) determining how many different kinds of carbon are in the moleculearrow_forward6 D 2. (1 pt) Limonene can be isolated by performing steam distillation of orange peel. Could you have performed this experiment using hexane instead of water? Explain. 3. (2 pts) Using GCMS results, analyze and discuss the purity of the Limonene obtained from the steam distillation of orange peel.arrow_forwardPart III. Arrange the following carbons (in blue) in order of increasing chemical shift. HO B NH 2 A CIarrow_forward
- 6. Choose the compound that will produce the spectrum below and assign the signals as carbonyl, aryl, or alkyl. 100 ō (ppm) 50 0 7. 200 150 Assign all of the protons on the spectrum below. 8. A B 4 E C 3 ō (ppm) 2 1 0 Choose the compound that will produce the spectrum below and assign the signals to the corresponding protons. OH 6 OH 3 2 1 0 4 ō (ppm)arrow_forwardIn the Thermo Fisher application note about wine analysis (Lesson 3), the following chromatogram was collected of nine components of wine. If peak 3 has a retention time of 3.15 minutes and a peak width of 0.070 minutes, and peak 4 has a retention time of 3.24 minutes and a peak width of 0.075 minutes, what is the resolution factor between the two peaks? [Hint: it will help to review Lesson 2 for this question.] MAU 300 200 T 34 5 100- 1 2 CO 6 7 8 9 0 2.4 2.6 2.8 3.0 3.2 3.4 3.6 3.8 4.0 4.2 4.4 4.6 4.8 5.0 5.2 Minutes 3.22 0.62 1.04 O 1.24arrow_forwardThe diagram shows two metals, A and B, which melt at 1000°C and 1400°C. State the weight percentage of the primary constituent (grains of C) that would be obtained by solidifying a 20% alloy of B. 1000°C a+L L+C 900°С 12 α a+C 45 1200 C L+y 140096 C+Y a+ß 800°C 700°C C+B 96 92 a+B 0 10 20 30 40 50 60 70 80 90 100 A % peso B Barrow_forward
- 8. Choose the compound that will produce the spectrum below and assign the signals to the corresponding protons. 2 4 3 ō (ppm) OH 4 6 6 СОН 2 1 0arrow_forward7. Assign all of the protons on the spectrum below. A B 2 C E 2 1 3 6 4 3 2 1 0arrow_forwarde. If (3R,4R)-3,4-dichloro-2,5-dimethylhexane and (3R,4S)-3,4-dichloro-2,5-dimethylhexane are in a solution at the same concentration, would this solution be expected to rotate plane polarized light (that is, be optically active)? Please provide your reasoning for your answer. [If you read this problem carefully, you will not need to draw out the structures to arrive at your answer...]arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

