
Concept explainers
12-23 Draw a structural formula for each compound.
- 3-Chloropropene
- 3-Methylcyclohexene
- 1,2-Dimethylcyclohexene
- /runs-3,4-Dimethyl-3-heptene
- Cydopropene
- 3-Hexyne
(a)
Interpretation:
To draw the structural formula for the compound.
Concept Introduction:
Structural formula is the spatial arrangement of atoms of a molecule in space.
In the condensed formula is a line-angle formula, the symbol for carbon and hydrogen atoms are not shown. Only carbon-carbon bonds are shown using lines. Single bonds are shown as  double bond as
double bond as  and triple bond as
and triple bond as  . The carbon atoms are represented as vertices and end of lines.
. The carbon atoms are represented as vertices and end of lines.
Answer to Problem 13P
Explanation of Solution
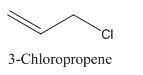
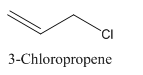
3-Chloropropene.
Carbon atoms in the longest chain are 3.
-ene means a double bond is present at Carbon 1.
Chlorine at Carbon 3.
Structure.
(b)
Interpretation:
To draw the structural formula for the compound.
Concept Introduction:
Structural formula is the spatial arrangement of atoms of a molecule in space. In the condensed formula is a line-angle formula, the symbol for carbon and hydrogen atoms are not shown. Only carbon-carbon bonds are shown using lines. Single bonds are shown as  double bond as
double bond as  and triple bond as
and triple bond as  . The carbon atoms are represented as vertices and end of lines.
. The carbon atoms are represented as vertices and end of lines.
Answer to Problem 13P
Explanation of Solution
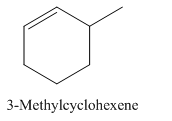
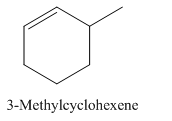
3-Methylcyclohexene.
Carbons in longest chain=6.
Cyclo means a cyclic hydrocarbon.
-ene means a double bond is present at Carbon 1.
Methyl at Carbon 3.
Structure.
(c)
Interpretation:
To draw the structural formula for the compound.
Concept Introduction:
Structural formula is the spatial arrangement of atoms of a molecule in space. In the condensed formula is a line-angle formula, the symbol for carbon and hydrogen atoms are not shown. Only carbon-carbon bonds are shown using lines. Single bonds are shown as  double bond as
double bond as 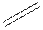 and triple bond as
and triple bond as  The carbon atoms are represented as vertices and end of lines.
The carbon atoms are represented as vertices and end of lines.
Answer to Problem 13P
Explanation of Solution
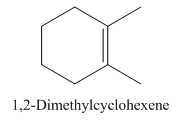
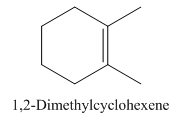
1,2-Dimethylcyclohexene.
Carbons in longest chain=6.
Cyclo means a cyclic hydrocarbon.
-ene means a double bond is present at Carbon 1.
Methyl at Carbon 1 and 2.
Structure.
(d)
Interpretation:
To draw the structural formula for the compound.
Concept Introduction:
Structural formula is the spatial arrangement of atoms of a molecule in space. In the condensed formula is a line-angle formula, the symbol for carbon and hydrogen atoms are not shown. Only carbon-carbon bonds are shown using lines. Single bonds are shown as  double bond as
double bond as  and triple bond as
and triple bond as  The carbon atoms are represented as vertices and end of lines.
The carbon atoms are represented as vertices and end of lines.
Answer to Problem 13P
Explanation of Solution
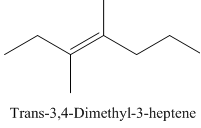
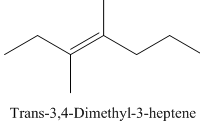
Trans-3,4-Dimethyl-3-heptene.
Carbons in longest chain=7.
-ene means a double bond is present at Carbon3.
Methyl at Carbon 3 and 4.
Trans isomer that means the two methyl are opposite in direction.
Structure.
(e)
Interpretation:
To draw the structural formula for the compound.
Concept Introduction:
Structural formula is the spatial arrangement of atoms of a molecule in space. In the condensed formula is a line-angle formula, the symbol for carbon and hydrogen atoms are not shown. Only carbon-carbon bonds are shown using lines. Single bonds are shown as  double bond as
double bond as 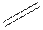 and triple bond as
and triple bond as  . The carbon atoms are represented as vertices and end of lines.
. The carbon atoms are represented as vertices and end of lines.
Answer to Problem 13P

Explanation of Solution
Cyclopropene.
Carbons in longest chain=3.
-ene means a double bond is present at Carbon1.
Cyclo means a cyclic hydrocarbon.
Structure.

(f)
Interpretation:
To draw the structural formula for the compound.
Concept Introduction:
Structural formula is the spatial arrangement of atoms of a molecule in space. In the condensed formula is a line-angle formula, the symbol for carbon and hydrogen atoms are not shown. Only carbon-carbon bonds are shown using lines. Single bonds are shown as  double bond as
double bond as 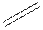 and triple bond as
and triple bond as  . The carbon atoms are represented as vertices and end of lines.
. The carbon atoms are represented as vertices and end of lines.
Answer to Problem 13P
Explanation of Solution
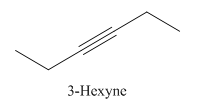
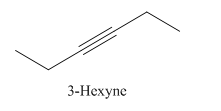
3-Hexyne.
Carbons in longest chain=6.
-yne means a triple bond is present at Carbon3.
Structure.
Want to see more full solutions like this?
Chapter 12 Solutions
Introduction To General, Organic, And Biochemistry
- in the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forwardMolecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forward
- Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forward
- What characteristics should an interface that forms an electrode have?arrow_forwardFor a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



