
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.50AP
What are the IUPAC names of the following
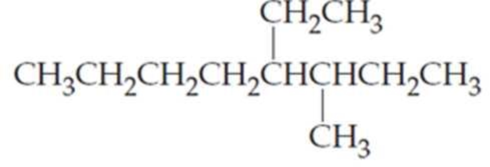
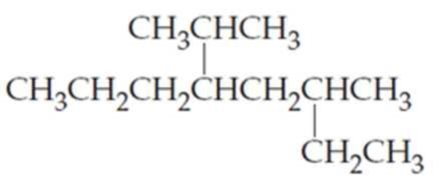
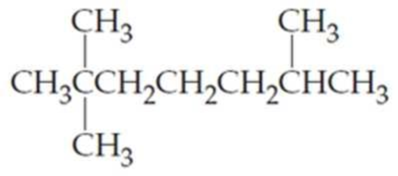
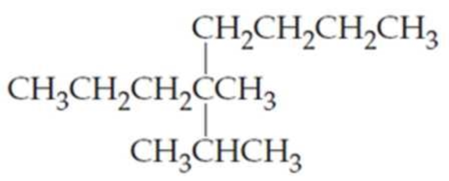
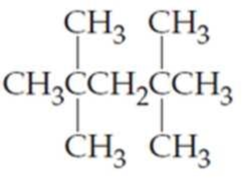
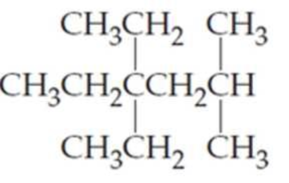
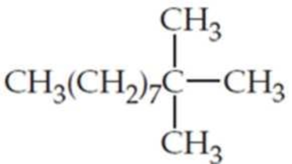
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4. (15 points) Consider a spherical organism of radius ro within
which respiration occurs at a uniform volumetric rate of That is,
oxygen (species A) consumption is governed by a first- order
reaction, homogeneous chemical reaction.
a. If a molar concentration of CA(ro) = CA,o is maintained at
the surface of the organism, obtain an expression for the
radial distribution of oxygen, CA(r), within the organism.
Hint: To simplify solution of the species diffusion equation,
invoke the transformation y = rCA.
b. Obtain an expression for the rate of oxygen consumption
within the organism.
c. Consider an organism of radius ro = 0.10 mm and a
diffusion coefficient of DAB = 108 m2/s. If CA, o = 5 x105
kmol/m3 and k1 20 s1, estimate the corresponding value
of the molar concentration at the center of the organism.
What is the rate of oxygen consumption by the organism?
3. (15 points) Living cells homogeneously distributed
(immobilized) with an agarose gel require glucose to survive.
An important aspect of the biochemical system design is the
effective diffusion coefficient of glucose (A) into the cell-
immobilized gel. Consider the experiment shows below where
a slab of the cell-immobilized gel of 1.0cm thickness is placed
within a well-mixed aqueous solution of glucose maintained at
a concentration of 50 mmol/L. The glucose consumption
within the cell-immobilized gel proceeds by a zero-order
process given by R₁ = -0.05 mmol/(L min). The solubilities of
glucose in both the water and the gel are the same; that is, the
concentration of the glucose on the water side of the water-gel
interface is equal to the concentration of the glucose on the gel
side of the water gel interface. A syringe is mounted at the
center of the gel carefully excises a tiny sample of the gel for
glucose analysis.
A
Well mixed solution
Constant
concentration
50nmol/L
Living…
Two tetrapeptides were isolated from a possum's sweat glands. These peptides were sequenced using Edman degradation and the following 2 sequences were obtained:
Gly-Asp-Ala-Leu
Gly-Asp-Asp-Leu
Can you please help show the titration curve for both of these peptides and calculate the PI?
Chapter 12 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 12.2 - Locate and identify the functional groups in (a)...Ch. 12.2 - Draw structures for molecules that fit the...Ch. 12.3 - Prob. 12.3PCh. 12.3 - There are two branched-chain isomers with the...Ch. 12.4 - Draw the following three isomers of C5H12 as...Ch. 12.4 - Prob. 12.6PCh. 12.4 - Prob. 12.7PCh. 12.4 - Draw both condensed and line structures for the...Ch. 12.5 - Which of the following structures represent the...Ch. 12.5 - Are the pairs of compounds shown below the same...
Ch. 12.6 - Identify each carbon in the molecule shown in...Ch. 12.6 - Prob. 12.12PCh. 12.6 - Prob. 12.13PCh. 12.6 - Draw and name alkanes that meet the following...Ch. 12.6 - Prob. 12.15KCPCh. 12.6 - Prob. 12.1CIAPCh. 12.6 - Prob. 12.2CIAPCh. 12.6 - Prob. 12.3CIAPCh. 12.8 - Prob. 12.1MRPCh. 12.8 - Prob. 12.2MRPCh. 12.8 - Prob. 12.16PCh. 12.8 - Write the structures of all singly chlorinated...Ch. 12.10 - Prob. 12.18PCh. 12.10 - Prob. 12.19PCh. 12.10 - What is wrong with the following names? It will be...Ch. 12.10 - Prob. 12.21KCPCh. 12.10 - Prob. 12.4CIAPCh. 12.10 - (a) What common produce items might you see...Ch. 12 - Convert the following models into line drawings...Ch. 12 - Prob. 12.23UKCCh. 12 - Prob. 12.24UKCCh. 12 - Give the IUPAC names for the following...Ch. 12 - Prob. 12.26UKCCh. 12 - What characteristics of carbon make possible the...Ch. 12 - Prob. 12.28APCh. 12 - Prob. 12.29APCh. 12 - Prob. 12.30APCh. 12 - For each of the following, give an example of a...Ch. 12 - Identify the highlighted functional groups in the...Ch. 12 - Identify the functional groups in the following...Ch. 12 - Propose structures for molecules that fit the...Ch. 12 - Prob. 12.35APCh. 12 - What requirement must be met for two compounds to...Ch. 12 - Prob. 12.37APCh. 12 - Prob. 12.38APCh. 12 - Prob. 12.39APCh. 12 - Prob. 12.40APCh. 12 - Give an example of a compound that meets the...Ch. 12 - (a)There are two isomers with the formula C4H10....Ch. 12 - Write condensed structures for the following...Ch. 12 - Prob. 12.44APCh. 12 - Prob. 12.45APCh. 12 - Which of the following pairs of structures are...Ch. 12 - Prob. 12.47APCh. 12 - Prob. 12.48APCh. 12 - Prob. 12.49APCh. 12 - What are the IUPAC names of the following alkanes?Ch. 12 - Prob. 12.51APCh. 12 - Write condensed structures for the following...Ch. 12 - Draw line structures for the following...Ch. 12 - Name the following cycloalkanes:Ch. 12 - Prob. 12.55APCh. 12 - Prob. 12.56APCh. 12 - Prob. 12.57APCh. 12 - Prob. 12.58APCh. 12 - Prob. 12.59APCh. 12 - Prob. 12.60APCh. 12 - Prob. 12.61APCh. 12 - Write the formulas of the four singly chlorinated...Ch. 12 - Write the formulas of the three doubly brominated...Ch. 12 - Identify the indicated functional groups in the...Ch. 12 - The line structure for pregabalin (Lyrica) is...Ch. 12 - Prob. 12.66CPCh. 12 - Prob. 12.67CPCh. 12 - Most lipsticks are about 70% castor oil and wax....Ch. 12 - Prob. 12.69CPCh. 12 - Prob. 12.70CPCh. 12 - Prob. 12.71CPCh. 12 - Which of the following structures represent the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Two tetrapeptides were isolated from a possum's sweat glands. These peptides were sequenced using Edman degradation and the following 2 sequences were obtained: Gly-Asp-Ala-Leu Gly-Asp-Asp-Leu What is the structure of the PTH derivative produced during the last round of amino acid sequencing?arrow_forwardWhat is the primary sequence of this undecapeptide? Also, if x-ray crystallography shows a highly stable hairpin turn within the polypeptide, what about the primary sequence explains this structural feature?arrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. H H ⚫OH HO- -H H- -OH H- -OH CH2OH Ag*, NH4OH, H2O Draw Fischer Projectionarrow_forward
- Draw the product of this reaction. Ignore inorganic byproducts. H₂O -OH H ⚫OH HO H HO- CH2OH Cu2+ Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. H、 H -OH H ⚫OH H -OH CH2OH Fehlings' solution ⑤ Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. HO C=0 H ⚫OH H ⚫OH HO- H HO H CH2OH Tollens' solution Draw Fischer Projectionarrow_forward
- Draw the product of this reaction. Ignore inorganic byproducts. H-C=O HO H HO H H- ⚫OH HO H CH2OH HNO3, H2O Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. HO HO- HO H HO ∙H HO CH2OH NaBH4, CH3OH Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. Но сво HO H HO H H OH H -OH CH2OH H2 Pd Draw Fischer Projectionarrow_forward
- Draw the Haworth projection for Gulose-ẞ-1,6-sorbose and answer the following questions. (Gulose will be in the pyranose form and Sorbose will be in the furanose form) a. Label the reducing and nonreducing ends of the disaccharide b. Label the glycosidic bond c. Circle the anomeric carbons and label them as hemiacetals or acetals. d. Can this disaccharide undergo mutarotation?arrow_forwardDraw the product of the reaction below. Ignore inorganic byproducts. H OH HO HO HO ·H H OH H OH excess CH3CH2I KOHarrow_forwardDraw the Haworth structures for the following: a. α-D-Gulopyranose b. ẞ-D-Sorbofuranose c. The two possible isomers of a-D-altrose (furanose and pyranose forms)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning

Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College




Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax
Biodiversity hotspots and functional diversity; Author: Stockholm Resilience Centre TV;https://www.youtube.com/watch?v=Gr_eIsFOKr4;License: Standard Youtube License