
An excellent way to make highly pure nickel metal for use in specialized steel alloys is to decompose Ni(CO)4 by heating it in a vacuum to slightly above room temperature.
Ni(CO)4(g) → Ni(s) + 4 CO(g)
The reaction is proposed to occur in four steps, the first of which is
Ni(CO)4(g) → Ni(CO)3(g) + CO(g)
Kinetic studies of this first-order decomposition reaction have been carried out between 47.3 °C and 66.0 °C to give the results in the table.*
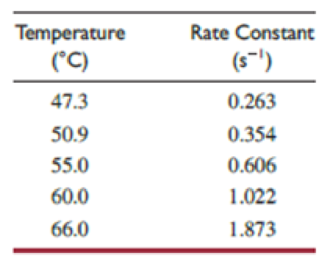
- (a) Determine the activation energy for this reaction.
- (b) Ni(CO)4 is formed by the reaction of nickel metal with carbon monoxide. Suppose that 2.05 g CO is combined with 0.125 g nickel metal. Determine the maximum mass (g) of Ni(CO)4 that can be formed.
Replacement of CO by another molecule in Ni(CO)4 was studied in the nonaqueous solvents toluene and hexane to understand the general principles that govern the chemistry of such compounds.*
Ni(CO)4(g) + P(CH3)3 → Ni(CO)3P(CH3)3 + CO
A detailed study of the kinetics of the reaction led to the mechanism
- (c) Which step in the mechanism is unimolecular? Which is bimolecular?
- (d) Add the steps of the mechanism to show that the result is the balanced equation for the observed reaction.
- (e) Is there an intermediate in this reaction? If so, what is it?
- (f) It was found that doubling the concentration of Ni(CO)4 increased the reaction rate by a factor of 2. Doubling the concentration of P(CH3)3 had no effect on the reaction rate. Based on this information, write the rate equation for the reaction.
- (g) Does the experimental rate equation support the proposed mechanism? Why or why not?
(a)
Interpretation:
The activation energy of the reaction has to be determined. The reaction is given below.
Concept Introduction:
The Arrhenius equation is given below.
Where,
By taking
The above equation is in the form of
Explanation of Solution
A table has to be made as shown below by using the given data.
A graph can be plotted as
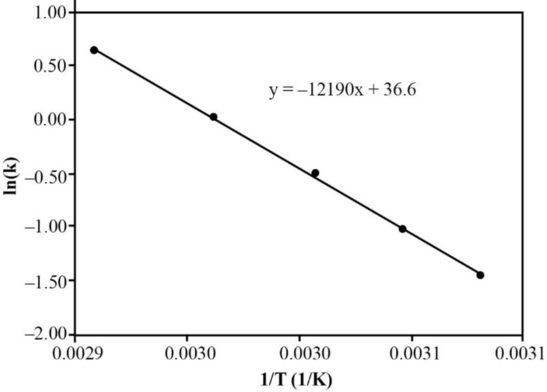
Figure
From the slope of the above graph, the activation energy of the reaction can be calculated.
Therefore, the activation energy of the reaction is
(b)
Interpretation:
The maximum mass
Explanation of Solution
The reverse reaction of the given reaction represents the formation of
Four moles of
Determination of limiting reagent:
The number of
The mass of
Therefore, the maximum mass
(c)
Interpretation:
The unimolecular and bimolecular step have to be chosen from the given mechanism.
Explanation of Solution
A reaction is called as elementary and unimolecular reaction if the reaction involves only one reactant. If the reaction has exactly two reactants, either two of the same reactants or one of each of different reactants, then the reaction is bimolecular and elementary.
The first step is unimolecular as it involves exactly one reactant. The second step is bimolecular as it involves exactly two reactants.
(d)
Interpretation:
The steps of the given mechanism has to be added in order to show that the result is the balanced equation for the observed reaction.
Explanation of Solution
The given mechanisms can be added as given below.
The resulting equation is the same as that given for the observed reaction.
(e)
Interpretation:
The intermediate involved in the reaction has to be determined.
Explanation of Solution
An intermediate is a chemical substance that is generated in an early step of a reaction and then used up in a later step. In the given mechanism, the intermediate is
(f)
Interpretation:
Doubling the concentration of
Explanation of Solution
The reaction is given below.
Suppose the rate of the above reaction is as follows,
Where,
Given that, doubling the concentration of
The second condition is doubling the concentration of
Now, the rate equation can be written as shown below.
(g)
Interpretation:
The experimental rate equation support the proposed mechanism or not, has to be explained. The mechanism is given below.
Explanation of Solution
The experimental rate equation can be written as shown below.
The slowest step in a mechanism is the rate determining step. Thus, according to the mechanism, the rate determining step is the first step. The rate equation can be written as follows,
Thus, the experimental rate equation supports the proposed mechanism.
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry: The Molecular Science
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





