
Nitryl fluoride is an explosive compound that can be made by oxidizing nitrogen dioxide with fluorine:
2 NO2(g) + F2(g) → 2 NO2F(g)
Several kinetics experiments, all done at the same temperature and involving formation of nitryl fluoride, are summarized in this table:
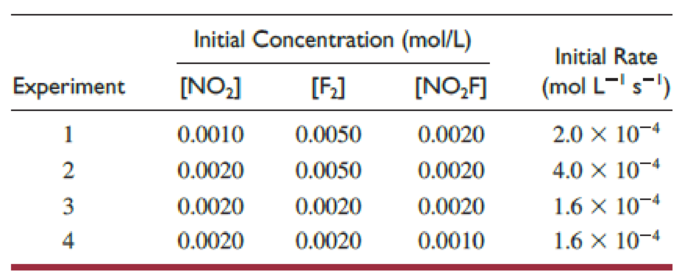
- (a) Write the rate law for the reaction.
- (b) Determine what the order of the reaction is with respect to each reactant and each product.
- (c) Calculate the rate constant k and express it in appropriate units.
(a)
Interpretation:
The rate law for the reaction has to be written.
Explanation of Solution
Suppose the rate law of the above reaction is as follows,
Where,
Carefully examining the table, it can be said that
Now, on dividing both the equations, the value of j can be found out.
Similarly, carefully examining the table, it can be said that
Now, on dividing both the equations, the value of i can be found out.
Similarly, carefully examining the table, it can be said that
Now, on dividing both the equations, the value of h can be found out.
Therefore, the rate law for the reaction is
(b)
Interpretation:
The order of the reaction with respect to each reactant and each product has to be determined.
Explanation of Solution
The rate law for the reaction is
Therefore, the order of the reaction with respect to
(c)
Interpretation:
The rate constant
Answer to Problem 95QRT
The average of the rate constant is
Explanation of Solution
The rate constant can be expressed as shown below.
For first experiment the rate constant can be calculated by plugging all the data given in the table.
In the first experiment, the initial concentration of
Now, the rate constant for first experiment is given below.
Similarly, the rate constant for rest of the experiments can be calculated. The calculated rate constant values are given in the table below.
|
Initial rate |
Rate constant | ||
The average of the rate constant is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry: The Molecular Science
- "Water gas" is an industrial fuel composed of a mixture of carbon monoxide and hydrogen gases. When this fuel is burned, carbon dioxide and water result. From the information given below, write a balanced equation and determine the enthalpy of this reaction: CO(g) + O2(g) → CO₂(g) + 282.8 kJ H2(g) + O2(g) → H₂O(g) + 241.8 kJ MacBook Airarrow_forwardPage of 3 4. Calculate AG for the following reaction at 25°C. Will the reaction occur (be spontaneous)? How do you know? NH3(g) + HCl(g) → NH4Cl(s) AH=-176.0 kJ AS-284.8 J-K-1arrow_forwardtrue or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 5. 4NO2(g) ⇔ 2N2O4(g)arrow_forward
- true or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 0.4. 2N2O4(g) ⇔ 4NO2(g)arrow_forwardtrue or false Using the following equilibrium, if heat is added the equilibrium will shift toward the reactants. N2(g) + 3H2(g) ⇔ 2NH3(g) + heatarrow_forwardTrue or False Using the following equilibrium, if heat is added the equilibrium will shift toward the products. N2O4(g) + heat ⇔ 2NO2(g)arrow_forward
- true or false Using the following equilibrium, if solid carbon is added the equilibrium will shift toward the products. C(s) + CO2(g) ⇔ 2CO(g)arrow_forwardProvide the complete mechanism for the reaction below. You must include appropriate arrows,intermediates, and formal charges. Please also provide a reason to explain why the 1,4-adduct is preferred over the 1,3-adduct.arrow_forwardWhich of the following pairs are resonance structures of one another? I. III. || III IV + II. :0: n P !༠ IV. EN: Narrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





