
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
4th Edition
ISBN: 9781265982959
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 46P
Albuterol (trade names Proventil and Ventolin) is a bronchodilator, a drug that widens airways, thus making it an effective treatment for individuals suffering from asthma. Draw a complete structure for albuterol with all atoms and lone pairs.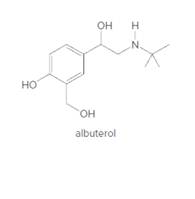
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
None
None
None
Chapter 11 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
Ch. 11.1 - Prob. 11.1PCh. 11.2 - Fill in all H's and lone pairs in each compound.Ch. 11.3 - Prob. 11.2PPCh. 11.3 - Prob. 11.2PCh. 11.3 - Prob. 11.3PCh. 11.3 - Prob. 11.3PPCh. 11.3 - How many lone pairs are present in lidocaine, the...Ch. 11.4 - Convert each compound to a condensed formula.Ch. 11.4 - Convert each condensed formula to a complete...Ch. 11.4 - Convert each skeletal structure to a complete...
Ch. 11.4 - Prob. 11.5PCh. 11.4 - How many H’s are bonded to each indicated carbon...Ch. 11.4 - Using the skeletal structure, determine the...Ch. 11.5 - Prob. 11.7PCh. 11.5 - Prob. 11.8PCh. 11.5 - For each compound. [1] Identify the functional...Ch. 11.5 - How do a carboxylic acid and an alcohol differ?...Ch. 11.5 - Label each of the following condensed structures...Ch. 11.5 - Prob. 11.11PCh. 11.5 - Prob. 11.12PCh. 11.5 - Identify all of the functional groups in atenolol,...Ch. 11.5 - Prob. 11.13PCh. 11.5 - Prob. 11.10PPCh. 11.5 - Prob. 11.14PCh. 11.6 - Indicate the polar bonds in each compound. Label...Ch. 11.6 - Prob. 11.11PPCh. 11.6 - Prob. 11.16PCh. 11.6 - Predict the water solubility of each compound.Ch. 11.6 - Prob. 11.17PCh. 11.7 - Prob. 11.18PCh. 11.7 - Prob. 11.19PCh. 11.7 - Prob. 11.20PCh. 11 - Prob. 21PCh. 11 - Prob. 22PCh. 11 - Complete each structure by filling in all H’s and...Ch. 11 - Complete the structure of mepivacaine by filling...Ch. 11 - Prob. 25PCh. 11 - Prob. 26PCh. 11 - Prob. 27PCh. 11 - Prob. 28PCh. 11 - “Ecstasy” is a widely used illegal stimulant....Ch. 11 - Prob. 30PCh. 11 - Explain why each C—C—C bond angle in benzene...Ch. 11 - Prob. 32PCh. 11 - Convert each compound to a condensed structure.Ch. 11 - Convert each compound to a condensed structure.Ch. 11 - Convert each compound to a skeletal structure.Ch. 11 - Convert each compound to a skeletal structure.Ch. 11 - Convert each shorthand structure to a complete...Ch. 11 - Convert each shorthand structure to a complete...Ch. 11 - Convert each skeletal structure to a complete...Ch. 11 - Convert each skeletal structure to a complete...Ch. 11 - A and B are ball-and-stick models of two compounds...Ch. 11 - Prob. 42PCh. 11 - What is wrong in each of the following shorthand...Ch. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Albuterol (trade names Proventil and Ventolin) is...Ch. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - (a) Identify the functional groups in donepezil,...Ch. 11 - Prob. 51PCh. 11 - GHB is an addictive, illegal recreational drug...Ch. 11 - Prob. 53PCh. 11 - Prob. 54PCh. 11 - Prob. 55PCh. 11 - Prob. 56PCh. 11 - Prob. 57PCh. 11 - (a) Identify the functional groups in venlafaxine,...Ch. 11 - You are given two unlabeled bottles of solids, one...Ch. 11 - State how potassium iodide (KI) and pentane...Ch. 11 - The given beaker contains 100 mL of the organic...Ch. 11 - Prob. 62PCh. 11 - Why do we need to know the shape of a molecule...Ch. 11 - 1,1-Dichloroethylene (CH2=CCl2) is a starting...Ch. 11 - Indicate the polar bonds in each molecule. Label...Ch. 11 - Indicate the polar bonds in each molecule. Label...Ch. 11 - Classify each molecule as polar or nonpolar.Ch. 11 - Classify each molecule as polar or nonpolar. a....Ch. 11 - Which molecule is more water soluble? Explain.Ch. 11 - Explain why pantothenic acid, vitamin B5, is water...Ch. 11 - Prob. 71PCh. 11 - Prob. 72PCh. 11 - Explain why regularly taking a large excess of a...Ch. 11 - You can obtain the minimum daily requirement of...Ch. 11 - Prob. 75PCh. 11 - Vitamin B6 is obtained by eating a diet that...Ch. 11 - Prob. 77PCh. 11 - Can an oxygen-containing organic compound, have...Ch. 11 - Prob. 79PCh. 11 - Prob. 80PCh. 11 - Benzocaine is the active ingredient in topical...Ch. 11 - Methyl salicylate is responsible for the...Ch. 11 - Answer the following questions about aldosterone,...Ch. 11 - Answer the following questions about...Ch. 11 - Prob. 85PCh. 11 - Skin moisturizers come in two types, (a) One type...Ch. 11 - THC is the active component in marijuana (Section...Ch. 11 - Cocaine is a widely abused, addicting drug....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the structure of 1-bromo-2-fluoroethane. Part 1 of 2 Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. ✡ ぬ Part 2 of 2 H H F Br H H ☑ Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. H F Br H Harrow_forwardPlease help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.arrow_forwardWhich of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forward
- Which of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forwardBased on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forwardPLEASE HELP URGENT!arrow_forward
- Draw the skeletal structure corresponding to the following IUPAC name: 7-isopropyl-3-methyldecanearrow_forwardWhich of the following oxyacids is the weakest? Group of answer choices H2SeO3 Si(OH)4 H2SO4 H3PO4arrow_forwardAdd conditions above and below the arrow that turn the reactant below into the product below in a single transformation. + More... If you need to write reagents above and below the arrow that have complex hydrocarbon groups in them, there is a set of standard abbreviations you can use. More... T H,N NC Datarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY