
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
4th Edition
ISBN: 9781265982959
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11.6, Problem 11.12PP
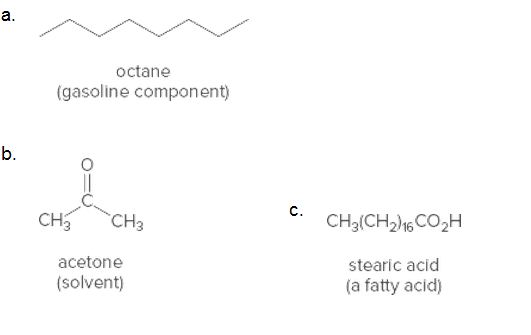
Predict the water solubility of each compound.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the structure of the DNA backbone?
PLEASE PLEASE PLEASE use hand drawn structures when poss
. M
1- MATCH each of the following terms to a structure from the list below. There is only one correct
structure for each term and structures may be used more than once. Place the letter of the structure
in the blank to the left of the corresponding term.
A. Sanger dideoxy method
C. Watson-Crick
B. GAUCGUAAA
D. translation
E. HOH2C
OH
OH
G. transcription
I. AUGGCUGAG
0
K. OPOH2C
0-
OH
N-
H
NH2
F. -OPOH2C
0-
OH
OH
H. Maxam-Gilbert method
J. replication
N
L.
HOH2C
a.
b.
C.
d.
e.
f.
g.
B
M. AGATCGCTC
a pyrimidine nucleoside
RNA base sequence with guanine at the 3' end.
DNA base sequence with cytosine at the 3' end.
a purine nucleoside
DNA sequencing method for the human genome
2'-deoxyadenosine 5'-phosphate
process by which mRNA directs protein synthesis
OH
NH2
Chapter 11 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
Ch. 11.1 - Prob. 11.1PCh. 11.2 - Fill in all H's and lone pairs in each compound.Ch. 11.3 - Prob. 11.2PPCh. 11.3 - Prob. 11.2PCh. 11.3 - Prob. 11.3PCh. 11.3 - Prob. 11.3PPCh. 11.3 - How many lone pairs are present in lidocaine, the...Ch. 11.4 - Convert each compound to a condensed formula.Ch. 11.4 - Convert each condensed formula to a complete...Ch. 11.4 - Convert each skeletal structure to a complete...
Ch. 11.4 - Prob. 11.5PCh. 11.4 - How many H’s are bonded to each indicated carbon...Ch. 11.4 - Using the skeletal structure, determine the...Ch. 11.5 - Prob. 11.7PCh. 11.5 - Prob. 11.8PCh. 11.5 - For each compound. [1] Identify the functional...Ch. 11.5 - How do a carboxylic acid and an alcohol differ?...Ch. 11.5 - Label each of the following condensed structures...Ch. 11.5 - Prob. 11.11PCh. 11.5 - Prob. 11.12PCh. 11.5 - Identify all of the functional groups in atenolol,...Ch. 11.5 - Prob. 11.13PCh. 11.5 - Prob. 11.10PPCh. 11.5 - Prob. 11.14PCh. 11.6 - Indicate the polar bonds in each compound. Label...Ch. 11.6 - Prob. 11.11PPCh. 11.6 - Prob. 11.16PCh. 11.6 - Predict the water solubility of each compound.Ch. 11.6 - Prob. 11.17PCh. 11.7 - Prob. 11.18PCh. 11.7 - Prob. 11.19PCh. 11.7 - Prob. 11.20PCh. 11 - Prob. 21PCh. 11 - Prob. 22PCh. 11 - Complete each structure by filling in all H’s and...Ch. 11 - Complete the structure of mepivacaine by filling...Ch. 11 - Prob. 25PCh. 11 - Prob. 26PCh. 11 - Prob. 27PCh. 11 - Prob. 28PCh. 11 - “Ecstasy” is a widely used illegal stimulant....Ch. 11 - Prob. 30PCh. 11 - Explain why each C—C—C bond angle in benzene...Ch. 11 - Prob. 32PCh. 11 - Convert each compound to a condensed structure.Ch. 11 - Convert each compound to a condensed structure.Ch. 11 - Convert each compound to a skeletal structure.Ch. 11 - Convert each compound to a skeletal structure.Ch. 11 - Convert each shorthand structure to a complete...Ch. 11 - Convert each shorthand structure to a complete...Ch. 11 - Convert each skeletal structure to a complete...Ch. 11 - Convert each skeletal structure to a complete...Ch. 11 - A and B are ball-and-stick models of two compounds...Ch. 11 - Prob. 42PCh. 11 - What is wrong in each of the following shorthand...Ch. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Albuterol (trade names Proventil and Ventolin) is...Ch. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - (a) Identify the functional groups in donepezil,...Ch. 11 - Prob. 51PCh. 11 - GHB is an addictive, illegal recreational drug...Ch. 11 - Prob. 53PCh. 11 - Prob. 54PCh. 11 - Prob. 55PCh. 11 - Prob. 56PCh. 11 - Prob. 57PCh. 11 - (a) Identify the functional groups in venlafaxine,...Ch. 11 - You are given two unlabeled bottles of solids, one...Ch. 11 - State how potassium iodide (KI) and pentane...Ch. 11 - The given beaker contains 100 mL of the organic...Ch. 11 - Prob. 62PCh. 11 - Why do we need to know the shape of a molecule...Ch. 11 - 1,1-Dichloroethylene (CH2=CCl2) is a starting...Ch. 11 - Indicate the polar bonds in each molecule. Label...Ch. 11 - Indicate the polar bonds in each molecule. Label...Ch. 11 - Classify each molecule as polar or nonpolar.Ch. 11 - Classify each molecule as polar or nonpolar. a....Ch. 11 - Which molecule is more water soluble? Explain.Ch. 11 - Explain why pantothenic acid, vitamin B5, is water...Ch. 11 - Prob. 71PCh. 11 - Prob. 72PCh. 11 - Explain why regularly taking a large excess of a...Ch. 11 - You can obtain the minimum daily requirement of...Ch. 11 - Prob. 75PCh. 11 - Vitamin B6 is obtained by eating a diet that...Ch. 11 - Prob. 77PCh. 11 - Can an oxygen-containing organic compound, have...Ch. 11 - Prob. 79PCh. 11 - Prob. 80PCh. 11 - Benzocaine is the active ingredient in topical...Ch. 11 - Methyl salicylate is responsible for the...Ch. 11 - Answer the following questions about aldosterone,...Ch. 11 - Answer the following questions about...Ch. 11 - Prob. 85PCh. 11 - Skin moisturizers come in two types, (a) One type...Ch. 11 - THC is the active component in marijuana (Section...Ch. 11 - Cocaine is a widely abused, addicting drug....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please use hand drawn structures when neededarrow_forwardB. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forwardBriefly answer three from the followings: a. What are the four structures of the protein? b. Why is the side chain (R) attached to the alpha carbon in the amino acids is important for the function? c. What are the types of amino acids? And how is it depend on the (R) structure? d. Write a reaction to prepare an amino acid. prodarrow_forward
- Answe Answer A and B pleasearrow_forward3. Refer to the data below to answer the following questions: Isoelectric point Amino Acid Arginine 10.76 Glutamic Acid 3.22 Tryptophan 5.89 A. Define isoelectric point. B. The most basic amino acid is C. The most acidic amino acid is sidizo zoarrow_forward3. A gas mixture contains 50 mol% H2 and 50 mol% He. 1.00-L samples of this gas mixture are mixed with variable volumes of O2 (at 0 °C and 1 atm). A spark is introduced to allow the mixture to undergo complete combustion. The final volume is measured at 0 °C and 1 atm. Which graph best depicts the final volume as a function of the volume of added O2? (A) 2.00 1.75 Final Volume, L 1.50 1.25 1.00 0.75 0.50 0.25 0.00 0.00 0.25 0.50 2.00 (B) 1.75 1.50 Final Volume, L 1.25 1.00 0.75 0.50- 0.25 0.00 0.75 1.00 0.00 0.25 Volume O₂ added, L 2 0.50 0.75 1.00 Volume O₂ added, L 2 2.00 2.00 (C) (D) 1.75 1.75 1.50 1.50 Final Volume, L 1.25 1.00 0.75 0.50 Final Volume, L 1.25 1.00 0.75 0.50 0.25 0.25 0.00 0.00 0.00 0.25 0.50 0.75 1.00 0.00 0.25 Volume O₂ added, L 0.50 0.75 1.00 Volume O₂ added, L 2arrow_forward
- Leucine is an essential amino acid with the systematic name 2-amino-3-methylpentanoic acid. It has pai 2.36 and pKa2 = 9.60. H2N-C(R)H-COOH and R is -CH2-CH(CH3)2 A. Draw the condensed structure for leucine, and label all chirality centers with an asterisk. B. How many possible stereoisomers of leucine are there? C. Draw a Fischer projection of L-leucine and label the chirality center(s) as R or S. D. What is the p/ of leucine? E. Draw the structure of the predominant form of leucine at 10.00. F. Draw the structure of the predominant form of leucine at pH = 1.50. G. Leucine is described as an essential amino acid. What does this mean? H. Show the alkyl halide you would use to prepare leucine by the amidomalonate method. =arrow_forwarda) Write out 6 completely different reactions of acetophenone (reagent, product). b) Write out 3 preparations of 1-methylcyclohexanol, using a different starting material for each one. You may use preps where you just change the functional group, and/or preps where you construct the carbon chain. c) Write out 3 preparations of 2-ethoxybenzoic acid, a different starting material for each one. You may use preps where you just change the functional group, and/or preps where you construct the carbon chain.arrow_forward12. CH3 OH OH H&C CH3 H₂C N OH H₂C CH3 H&C CH3 H₂C' CH3 H.C CH3OH H.C CH2CH3OH CH3CEN Which one of these 17 compounds is represented by this IR and this 'H NMR spectrum? IR Spectrum 3000 4000 3000 NMR Spectrum 2000 £500 RAVENUMBER 2000 1500 9 8 6 5 10 HP-00-290 ppm m 1000 500 1000 4 °arrow_forward
- Draw the structure of (E,6R) 6-methoxy-4-hepten-2-one. Give the IUPAC name of this compound, including stereochemistry. Draw the most stable chair conformation of (cis) 1,3-isobutylcyclohexane. H HC=CCH₂ CH2CH3 EN(CH3)2 -CN(CH3)2arrow_forward10. Write out the mechanism (intermediate/transition state) for this reaction; indicate stereochemistry in product. H3C CH₂OH CH3 SN1 Harrow_forwardWrite "most" under the member of each trio which is most stable. Write "least under the member of each trio which is least stable. b) Draw a Fischer projection of a pair of enantiomers with three chiral carbons. Which of these two would you expect to be more soluble in water? Why? 1-butanol 1-heptanol Which of these two would you expect to have the higher boiling point? Why? hexyl methyl ether 1-heptanolarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY