
Prescott's Microbiology
10th Edition
ISBN: 9781259281594
Author: Joanne Willey, Linda Sherwood Adjunt Professor Lecturer, Christopher J. Woolverton Professor
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.7, Problem 2MI
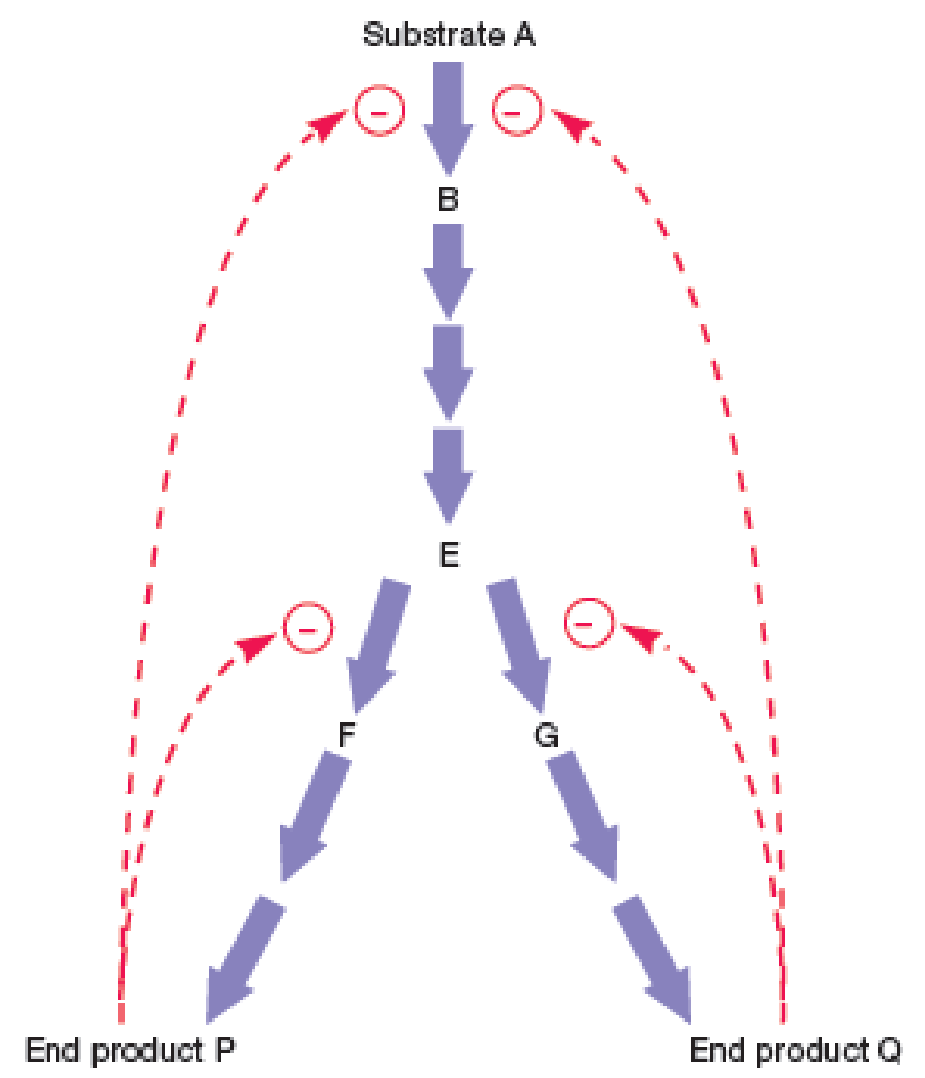
Figure 10.21 Feedback Inhibition. Feedback inhibition in a branching pathway with two end products. The branch-point enzymes, those catalyzing the conversion of intermediate E to F and G, are regulated by feedback inhibition. Products P and Q also inhibit the initial reaction in the pathway. A colored line with a minus sign at one end indicates that an end product, P or Q, is inhibiting the enzyme catalyzing the step next to the minus.
What would happen to product formation if P and Q inhibited the formation of intermediate E independently of one another?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Stage
Percent
Time in Hours
Interphase
.60
14.4
Prophase
.20
4.8
Metaphase
.10
2.4
Anaphase
.06
1.44
Telophase
.03
.72
Cytukinesis
.01
.24
Can you summarize the results in the chart and explain which phases are faster and why the slower ones are slow?
Can you circle a cell in the different stages of mitosis?
1.prophase
2.metaphase
3.anaphase
4.telophase
5.cytokinesis
Which microbe does not live part of its lifecycle outside humans?
A. Toxoplasma gondii
B. Cytomegalovirus
C. Francisella tularensis
D. Plasmodium falciparum
explain your answer thoroughly.
Chapter 10 Solutions
Prescott's Microbiology
Ch. 10.1 - Figure 10.2 The Relationship of G to the...Ch. 10.1 - What kinds of work are carried out in a cell?...Ch. 10.1 - What is thermodynamics? Summarize the first and...Ch. 10.1 - Define entropy and enthalpy. Do living cells...Ch. 10.1 - Prob. 4RIACh. 10.1 - Prob. 5RIACh. 10.2 - Why is ATP called a high-energy molecule? How is...Ch. 10.2 - Describe the energy cycle and ATPs role in it....Ch. 10.3 - Prob. 1MICh. 10.3 - Prob. 2MI
Ch. 10.4 - Figure 10.6 Electron Movement and Reduction...Ch. 10.4 - How is the direction of electron flow between...Ch. 10.4 - When electrons flow from the NAD+/NADH conjugate...Ch. 10.4 - Which among the following would be the best...Ch. 10.4 - In general terms, how is G related to E0? What is...Ch. 10.4 - Name and briefly describe the major electron...Ch. 10.6 - Will an enzyme with a relatively high Km have a...Ch. 10.6 - Prob. 2MICh. 10.6 - What is an apoenzyme? A holoenzyme? What are the...Ch. 10.6 - Illustrate the effect enzymes have on the...Ch. 10.6 - How does enzyme activity change with substrate...Ch. 10.6 - What special properties might an enzyme isolated...Ch. 10.6 - What are competitive and noncompetitive...Ch. 10.6 - How are enzymes and ribozymes similar? How do they...Ch. 10.7 - Figure 10.19 Allosteric Regulation. The structure...Ch. 10.7 - Figure 10.21 Feedback Inhibition. Feedback...Ch. 10.7 - Briefly describe the three ways a metabolic...Ch. 10.7 - Define the terms metabolic channeling and...Ch. 10.7 - Define allosteric enzyme and allosteric effector.Ch. 10.7 - Prob. 4RIACh. 10.7 - Prob. 5RIACh. 10.7 - What is the significance of the fact that...Ch. 10 - Examine the structures of macromolecules in...Ch. 10 - Most enzymes do not operate at their biochemical...Ch. 10 - Examine the branched pathway shown here for the...Ch. 10 - Prob. 4CHI
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Select all of the following that the ablation (knockout) or ectopoic expression (gain of function) of Hox can contribute to. Another set of wings in the fruit fly, duplication of fingernails, ectopic ears in mice, excess feathers in duck/quail chimeras, and homeosis of segment 2 to jaw in Hox2a mutantsarrow_forwardSelect all of the following that changes in the MC1R gene can lead to: Changes in spots/stripes in lizards, changes in coat coloration in mice, ectopic ear formation in Siberian hamsters, and red hair in humansarrow_forwardPleiotropic genes are genes that (blank) Cause a swapping of organs/structures, are the result of duplicated sets of chromosomes, never produce protein products, and have more than one purpose/functionarrow_forward
- A loss of function mutation in Pitx1 enhancers can cause (blank) Removal of Pitx1 exons and growth of ectopic hindlimbs, growth of extra ectopic forelimbs, loss of forelimb specification and development, and loss of hindlimb specification and developmentarrow_forwardHox1a most likely contributes to (blank) patterning in the developing embryo? Ventral, posterior, limb or anteriorarrow_forwardSelect all of the following that can help establish Hox gene expression boundaries (things that affect Hox and not things that Hox affects). Retinoic acid, anterior/posterior axis, fibroblast growth factors, vagal neural crest, and enhancersarrow_forward
- Ectopic expression of Hox often results in (blank) phenotypes. (Blank) transformations are characterized by the replacement of one body part/structure with another. Hoxeotic, homealoneotic, joexotic, or homeoticarrow_forwardWhat's the difference when drawing omega-6 and omega-3?arrow_forward. Consider a base substitution mutation that occurred in a DNA sequence that resulted in a change in the encoded protein from the amino acid glutamic acid to aspartic acid. Normally the glutamic acid amino acid is located on the outside of the soluble protein but not near an active site. O-H¨ A. What type of mutation occurred? O-H B. What 2 types of chemical bonds are found in the R-groups of each amino acid? The R groups are shaded. CH2 CH2 CH2 H2N-C-COOH H2N-C-COOH 1 H Glutamic acid H Aspartic acid C. What 2 types of bonds could each R-group of each of these amino acids form with other molecules? D. Consider the chemical properties of the two amino acids and the location of the amino acid in the protein. Explain what effect this mutation will have on this protein's function and why.arrow_forward
- engineered constructs that consist of hollow fibers are acting as synthetic capillaries, around which cells have been loaded. The cellular space around a single fiber can be modeled as if it were a Krogh tissue cylinder. Each fiber has an outside “capillary” radius of 100 µm and the “tissue” radius can be taken as 200 µm. The following values apply to the device:R0 = 20 µM/secaO2 = 1.35 µM/mmHgDO2,T = 1.67 x 10-5 cm2/secPO2,m = 4 x 10-3 cm/secInstead of blood inside the fibers, the oxygen transport and tissue consumption are being investigated by usingan aqueous solution saturated with pure oxygen. As a result, there is no mass transfer resistance in the synthetic“capillary”, only that due to the membrane itself. Rather than accounting for pO2 variations along the length ofthe fiber, use an average value in the “capillary” of 130 mmHg.Is the tissue fully oxygenated?arrow_forwardMolecular Biology Please help with question. thank you You are studying the expression of the lac operon. You have isolated mutants as described below. In the presence of glucose, explain/describe what would happen, for each mutant, to the expression of the lac operon when you add lactose AND what would happen when the bacteria has used up all of the lactose (if the mutant is able to use lactose).5. Mutations in the lac operator that strengthen the binding of the lac repressor 200 fold 6. Mutations in the promoter that prevent binding of RNA polymerase 7. Mutations in CRP/CAP protein that prevent binding of cAMP8. Mutations in sigma factor that prevent binding of sigma to core RNA polymerasearrow_forwardMolecular Biology Please help and there is an attached image. Thank you. A bacteria has a gene whose protein/enzyme product is involved with the synthesis of a lipid necessary for the synthesis of the cell membrane. Expression of this gene requires the binding of a protein (called ACT) to a control sequence (called INC) next to the promoter. A. Is the expression/regulation of this gene an example of induction or repression?Please explain:B. Is this expression/regulation an example of positive or negative control?C. When the lipid is supplied in the media, the expression of the enzyme is turned off.Describe one likely mechanism for how this “turn off” is accomplished.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning
Anaerobic Respiration; Author: Bozeman Science;https://www.youtube.com/watch?v=cDC29iBxb3w;License: Standard YouTube License, CC-BY