
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 20P
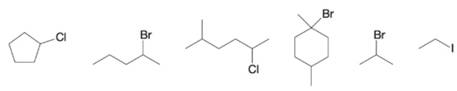
Which of the following compounds can be prepared by radical halogenations which little complication by formation of isomeric by-products?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
+ HCl →?
Draw the molecule on the canvas by choosing buttons from the Tools (for bonas), Atoms
and Advanced Template toolbars. The single bond is active by default.
+
M
C
+
H± 2D
EXP. CONT. K
?
L
1
H₁₂C
[1]
A
HCN O
S
CH3
CH 3
CI
Br
HC
H₂
CH
CH
CH3
-
P
F
SH
SH
0
Chapter 10 Solutions
Organic Chemistry
Ch. 10 - Prob. 1PPCh. 10 - Prob. 2PPCh. 10 - Practice Problem 10.3 How would the molecular ion...Ch. 10 - Prob. 4PPCh. 10 - Prob. 5PPCh. 10 - Prob. 6PPCh. 10 - Practice Problem 10.7 Chlorination reactions of...Ch. 10 - Prob. 8PPCh. 10 - Prob. 9PPCh. 10 - Prob. 10PP
Ch. 10 - Prob. 11PPCh. 10 - Practice Problem 10.12 Benzylic radicals, due to...Ch. 10 - Prob. 13PPCh. 10 - Practice Problem 10.14 Show how the following...Ch. 10 - Prob. 15PPCh. 10 - Prob. 16PPCh. 10 - Prob. 17PPCh. 10 - Prob. 18PCh. 10 - Explain the relative distribution of produces...Ch. 10 - 10.20 Which of the following compounds can be...Ch. 10 - Prob. 21PCh. 10 - Prob. 22PCh. 10 - Prob. 23PCh. 10 - Prob. 24PCh. 10 - 10.25 List in order of decreasing stability all of...Ch. 10 - Prob. 26PCh. 10 - Prob. 27PCh. 10 - Prob. 28PCh. 10 - Starting with the compound or compounds indicated...Ch. 10 - Prob. 30PCh. 10 - 10.31 Synthesize each of the following compounds...Ch. 10 - 10.32 Synthesize each of the following compounds...Ch. 10 - Prob. 33PCh. 10 - Prob. 34PCh. 10 - Prob. 35PCh. 10 - Write a mechanism for the following reaction.Ch. 10 - Prob. 37PCh. 10 - The halogen atom of an alkyl halide can be...Ch. 10 - Prob. 39PCh. 10 - Prob. 40PCh. 10 - If one were to try to draw the simplest Lewis...Ch. 10 - Prob. 1LGPCh. 10 - 2. (a) Propose a synthesis of 2-methoxypropene...Ch. 10 - Use the single-bond dissociation energies of Table...Ch. 10 - 10.2 In the radical chlorination of methane, one...Ch. 10 - Prob. 3QCh. 10 - Use the single-bond dissociation energies of Table...Ch. 10 - Prob. 5QCh. 10 - Prob. 6Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
47. A 250.0-mL buffer solution is 0.250 M in acetic acid and 0.250 M in sodium acetate.
a. What is the initial ...
Chemistry: A Molecular Approach (4th Edition)
The bioremediation process shown in the photograph is used to remove benzene and other hydrocarbons from soil c...
Microbiology: An Introduction
Examine the following diagrams of cells from an organism with diploid number 2n = 6, and identify what stage of...
Genetic Analysis: An Integrated Approach (3rd Edition)
SCIENTIFIC INQUIRY You are handed a mystery pea plant with tall stems and axial flowers and asked to determine ...
Campbell Biology (11th Edition)
8. A classic way to isolate thymidylate synthase—negative mutants of bacteria is to treat a growing culture wit...
Biochemistry: Concepts and Connections (2nd Edition)
The similarity and difference in ionic and covalent bond should be determined. Concept Introduction: The intera...
Living By Chemistry: First Edition Textbook
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Please consider the two all 'cis' isomers of trimethylcyclohexane drawn below. Draw the two chair conformers of each stereoisomer below (1 and 2) and calculate their torsional interaction energies in order to identify the lower energy conformer for each stereoisomer. Based on your calculations, state which of the two stereoisomers 1 and 2 is less stable and which is more stable. [1,3-diaxial CH3 CH3 = 3.7kcal/mol; 1,3-diaxial CH3 H = 0.88kcal/mol; cis-1,2 (axial:equatorial) CH3 CH3 = 0.88kcal/mol; trans-1,2-diequatorial CH3 CH3 = 0.88kcal/mol) all-cis-1,2,3- 1 all-cis-1,2,4- 2arrow_forwardNonearrow_forwardWhat is the mechanism by which the 1,4 product is created? Please draw it by hand with arrows and stuff.arrow_forward
- What is the relationship between A and B? H3C A Br Cl H3C B Br relationship (check all that apply) O same molecule O enantiomer O diastereomer structural isomer O stereoisomer isomer O need more information to decide O same molecule ☐ enantiomer Br Br Br CH3 Br CI CH3 O diastereomer ☐ structural isomer ☐ stereoisomer isomer O need more information to decide O same molecule O enantiomer Odiastereomer structural isomer O stereoisomer ☐ isomer O need more information to decidearrow_forwardb. Please complete the zig-zag conformation of the compound (3R,4S)-3,4-dichloro-2,5-dimethylhexane by writing the respective atoms in the boxes. 4arrow_forwardc. Serricornin, the female-produced sex pheromone of the cigarette beetle, has the following structure. OH What is the maximum number of possible stereoisomers? Is this structure a meso compound? d. Please consider the natural product alkaloids shown below. Are these two structures enantiomers, diastereomers or conformers? H HO H H HN HO HN R R с R=H cinchonidine R=ET cinchonine Harrow_forward
- Nail polish remover containing acetone was spilled in a room 5.23 m × 3.28 m × 2.76 m. Measurements indicated that 2,250 mg of acetone evaporated. Calculate the acetone concentration in micrograms per cubic meter.arrow_forwardPlease help me answer number 1. 1. If your graphs revealed a mathematical relationship between specific heat and atomic mass, write down an equation for the relationship. I also don't understand, is the equation from the line regression the one that I'm suppose use to show the relationship? If so could you work it all the way out?arrow_forwardDescribe the principle of resonance and give a set of Lewis Structures to illustrate your explanation.arrow_forward
- Don't used hand raitingarrow_forwardIt is not unexpected that the methoxyl substituent on a cyclohexane ring prefers to adopt the equatorial conformation. OMe H A G₂ = +0.6 kcal/mol OMe What is unexpected is that the closely related 2-methoxytetrahydropyran prefers the axial conformation: H H OMe OMe A Gp=-0.6 kcal/mol Methoxy: CH3O group Please be specific and clearly write the reason why this is observed. This effect that provides stabilization of the axial OCH 3 group in this molecule is called the anomeric effect. [Recall in the way of example, the staggered conformer of ethane is more stable than eclipsed owing to bonding MO interacting with anti-bonding MO...]arrow_forward206 Pb 82 Express your answers as integers. Enter your answers separated by a comma. ▸ View Available Hint(s) VAΣ ΜΕ ΑΣΦ Np, N₁ = 82,126 Submit Previous Answers ? protons, neutronsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License