
Concept explainers
(a)
Interpretation:
The increasing order of acidity of the alcohols is to be stated.
Concept introduction:
Acidity of a molecule is dependent on the value of pKa. This pKa value is known as the dissociation constant of an acid. The dissociation constant of an acid changes with the change in temperature. More is the value of pKa, lesser is the acidity of an acid. Strength of an acid also depends upon the stability of the anion generated after loosing a hydrogen ion.
Answer to Problem 10.46AP
The increasing order of acidity of the alcohols is given below.
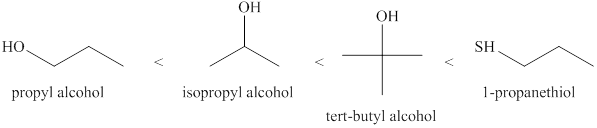
Explanation of Solution
Acidity of the molecules is dependent upon the stability of the ion formed after releasing hydrogen ion.
Sulfur atom present in 1- propanethiol is larger than the size of the oxygen atom. Therefore, negative charge present on sulfur atom is stabilized. Due to this 1- propanethiol is the strongest acid among the given molecules. Tertiary alcohols are more acidic than the secondary and primary alcohols. Therefore, tert−butyl alcohol is the stronger acid than isopropyl alcohol and propyl alcohol.
Order of the acidity is shown below in Figure 1.

Figure 1
The increasing order of acidity of the molecules is propyl alcohol<isopropyl alcohol<tert−butyl alcohol<propanethiol.
(b)
Interpretation:
The increasing order of acidity of the molecules is to be stated.
Concept introduction:
Acidity of a molecule is dependent on the value of pKa. This pKa value is known as the dissociation constant of an acid. The dissociation constant of an acid changes with the change in temperature. More is the value of pKa, lesser is the acidity of an acid. Strength of an acid also depends upon the stability of the anion generated after loosing a hydrogen ion.
Answer to Problem 10.46AP
The increasing order of acidity of the molecules is given below.
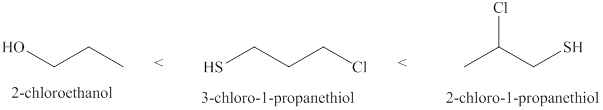
Explanation of Solution
Acidity of the molecules is dependent upon the stability of the ion formed after releasing hydrogen ion. Sulfur atom present in 3- chloro -1- propanethiol is larger than the size of the oxygen atom. Therefore, negative charge present on sulfur atom is stabilized. Due to this, 3- chloro -1- propanethiol is the strongest acid among the given molecules. Position of the electronegative atom also determines the strength of an acid. Closer the electronegative atom to the negative charge generated after releasing the hydrogen ion, more is the strength of the acid.
Therefore, increasing order of the given molecules is shown below in Figure 2.
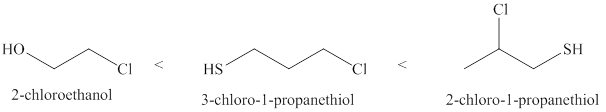
Figure 2
The increasing order of acidity of the given molecules is 2-chloroethanol<3-chloro-1-propanethiol<2-chloro- 1-propanethiol.
(c)
Interpretation:
The increasing order of acidity of the molecules is to be stated.
Concept introduction:
Acidity of a molecule is dependent on the value of pKa. This pKa value is known as the dissociation constant of an acid. The dissociation constant of an acid changes with the change in temperature. More is the value of pKa, lesser is the acidity of an acid. Strength of an acid also depends upon the stability of the anion generated after loosing a hydrogen ion.
Answer to Problem 10.46AP
The increasing order of acidity of the molecules is stated below.
CH3N¨H−CH2CH2CH2−O¨¨H<CH3N¨H−CH2CH2−O¨¨H<(CH3)3+N−CH2CH2−O¨¨H
Explanation of Solution
Acidity of the molecules is dependent upon the stability of the ion formed after releasing hydrogen ion. Nitrogen atom which is positively charged, stabilizes the negative charge which is generated after releasing the hydrogen ion. Therefore, structure 3 is the most stable structure with most acidic character. Position of the electronegative atom also determines the strength of an acid. Closer the electronegative atom to the generated negative charge after releasing the hydrogen ion, more is the strength of the acid.
Therefore, increasing order of the given molecules is stated below.
CH3N¨H−CH2CH2CH2−O¨¨H<CH3N¨H−CH2CH2−O¨¨H<(CH3)3+N−CH2CH2−O¨¨H
The increasing order of acidity of the given molecules is CH3N¨H−CH2CH2CH2−O¨¨H<CH3N¨H−CH2CH2−O¨¨H<(CH3)3+N−CH2CH2−O¨¨H.
(d)
Interpretation:
The increasing order of acidity of the molecules is to be stated.
Concept introduction:
Acidity of a molecule is dependent on the value of pKa. This pKa value is known as the dissociation constant of an acid. The dissociation constant of an acid changes with the change in temperature. More is the value of pKa, lesser is the acidity of an acid. Strength of an acid also depends upon the stability of the anion generated after loosing a hydrogen ion.
Answer to Problem 10.46AP
The increasing order of acidity of the molecules is stated below.
−:¨O¨−CH2CH2−O¨¨H<CH3−CH2CH2−O¨¨H<CH3¨O¨−CH2CH2−O¨¨H<CH3¨O¨−CH2CH2CH2−+O¨H
Explanation of Solution
Acidity of the molecules is dependent upon the stability of the ion formed after releasing the hydrogen ion. In structure 4, a neutral species is generated after releasing the H+ ion. Therefore, the molecule in structure 4 is the most acidic in nature. Position of the electronegative atom also determines the strength of an acid. Closer the electronegative atom to the generated negative charge after releasing the hydrogen ion, more is the strength of an acid. Therefore, molecule 3 is less acidic than molecule in structure 4. In structure 1, oxygen has already a negative charge. Therefore, it is already in its stabilized form and acts as a weakest acid among the given molecules.
Therefore, increasing order of acidity of the given molecules is stated below.
−:¨O¨−CH2CH2−O¨¨H<CH3−CH2CH2−O¨¨H<CH3¨O¨−CH2CH2−O¨¨H<CH3¨O¨−CH2CH2CH2−+O¨H
The increasing order of acidity of the given molecules is −:¨O¨−CH2CH2−O¨¨H<CH3−CH2CH2−O¨¨H<CH3¨O¨−CH2CH2−O¨¨H<CH3¨O¨−CH2CH2CH2−+O¨H
(e)
Interpretation:
The increasing order of acidity of the molecules is to be stated.
Concept introduction:
Acidity of a molecule is dependent on the value of pKa. This pKa value is known as the dissociation constant of an acid. The dissociation constant of an acid changes with the change in temperature. More is the value of pKa, lesser is the acidity of an acid. Strength of an acid also depends upon the stability of the anion generated after loosing a hydrogen ion.
Answer to Problem 10.46AP
The increasing order of acidity of the molecules is stated below.
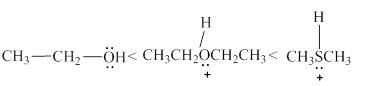
Explanation of Solution
Acidity of the molecules is dependent upon the stability of the ion formed after releasing the hydrogen ion. In structure 3, Size of the sulfur atom is more as compared to the size of the oxygen atom. The H+ ion is held loosely with the sulfur atom. Therefore, the molecule in structure 3 easily releases the hydrogen ion and becomes the most acidic in nature. In structure 2 and 3, a neutral species is generated after releasing the H+ ion Therefore, molecule 2 and 3 are more acidic than molecule 1.
Therefore, increasing order of the given molecules is stated below in Figure 3.
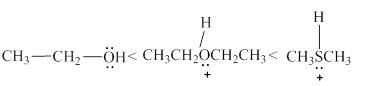
Figure 3
The increasing order of acidity of the given molecules is shown above in Figure 3.
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
- Identify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forward
- a. H3C CH3 H, 1.0 equiv. Br2arrow_forwardH3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forward
- in the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forwardIf we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


