
Concept explainers
(a)
Interpretation:
The given reaction is to be completed and explained to give the principal products.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The SN2 reaction is a nucleophilic substitution bimolecular single step reaction in which the addition of nucleophile and removal of leaving group takes place simultaneously.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
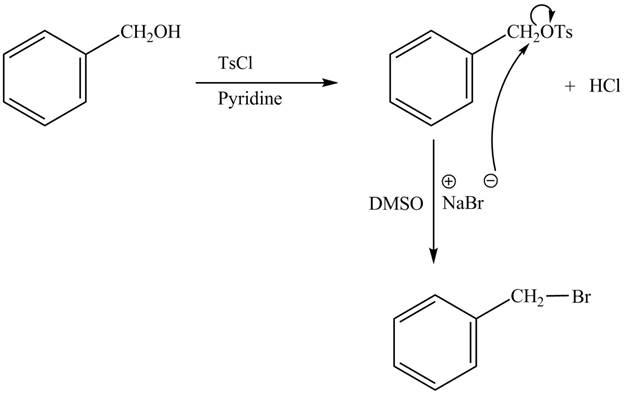
The tosyl chloride is used to make the hydroxide group a good leaving group by replacing its hydrogen with tosyl group. The −OTs group thus formed is good leaving group and is replaced by the bromide nucleophile easily to give the benzyl chloride as the product.
Explanation of Solution
The given reaction is shown below.
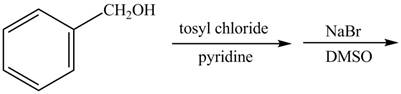
Figure 1
The complete reaction with the products is shown below.
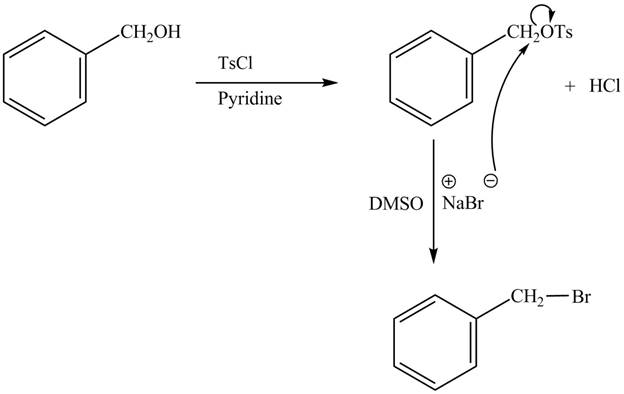
Figure 2
The reaction of the alcohols with tosyl chloride is the reaction to make the hydroxide group a good leaving group. The hydrogen is replaced by the tosyl group. The −OTs is good leaving group which is substituted by bromide ion from ![]() to give the halide. The product thus obtained in the end is benzyl bromide.
to give the halide. The product thus obtained in the end is benzyl bromide.
The completed reaction is shown in Figure 2.
(b)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The SN2 reaction is a nucleophilic substitution bimolecular reaction in which the addition of nucleophile and elimination of a leaving group takes place simultaneously. The rate of reaction depends upon both the substrate and reactant in the rate law equation.
Answer to Problem 10.59AP
The complete reaction is shown below.
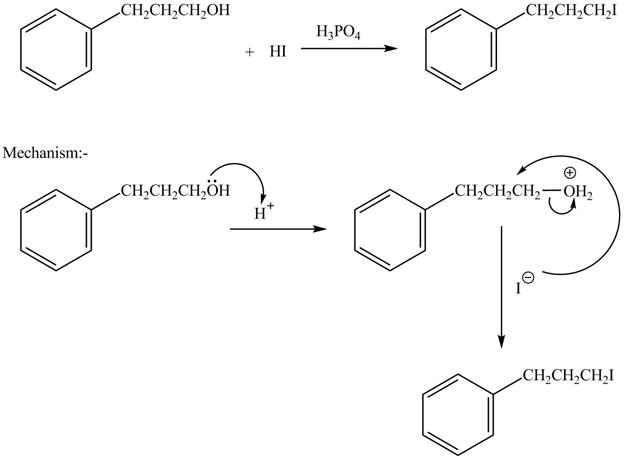
The acid is used to make the hydroxide group a good leaving group. The iodide group than substitutes the protonated hydroxide group to give halide product.
Explanation of Solution
The given reaction is shown below.
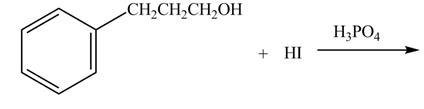
Figure 3
The complete reaction with the products is shown below.

Figure 4
The hydroxide group in alcohols is not a good leaving group in order to perform a nucleophilic substitution reaction on alcohols to produce more compounds. Hydroxide group is made a good leaving group by protonating the hydroxide group in the first step. After then the iodide ion attacks and eliminates protonated hydroxide group to halide product.
The completed reaction is shown in Figure 4.
(c)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The SN2 reaction is a nucleophilic substitution bimolecular reaction in which the addition of nucleophile and elimination of a leaving group takes place simultaneously. The rate of reaction depends upon both the substrate and reactant in the rate law equation.
Answer to Problem 10.59AP
The complete reaction is shown below.
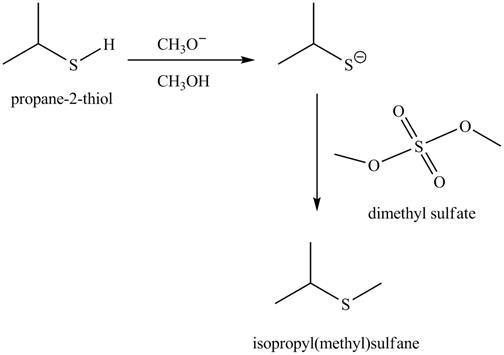
The acid-base reaction between the thiol group and methoxide ion takes place first to give sulfide ion. The sulfide ion then reacts with methylating agent dimethyl sulfate to give the methylated product isopropyl(methyl) sulfane.
Explanation of Solution
The given reaction is shown below.
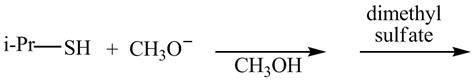
Figure 5
The complete reaction with the products is shown below.
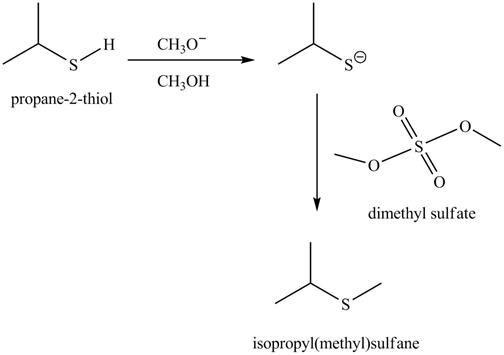
Figure 6
The methoxide ion acts as a base and takes away the hydrogen of the thiol group of propane-2-thiol to give the sulfide ion. This ion then reacts with the dimethyl sulfate to undergo methylation reaction to give the methylated product isopropyl(methyl) sulfane.
The completed reaction is shown in Figure 6.
(d)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The SN2 reaction is a nucleophilic substitution bimolecular reaction in which the addition of nucleophile and elimination of a leaving group takes place simultaneously. The rate of reaction depends upon both the substrate and reactant in the rate law equation.
Answer to Problem 10.59AP
The complete reaction is shown below.
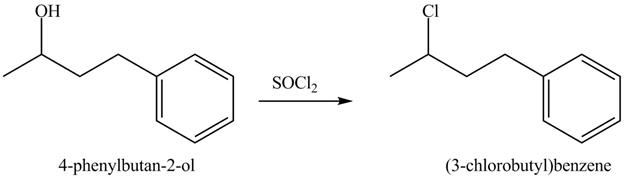
This is an SN2 reaction. Chloride group substitutes the hydroxide group.
Explanation of Solution
The given reaction is shown below.
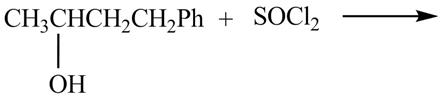
Figure 7
The complete reaction with the products is shown below.

Figure 8
The reaction of alcohols with thionyl chloride is a SN2 reaction. Chloride group substitutes the hydroxide group. Therefore, the 4-phenylbutan-2-ol reacts to give 3-chlorobutylbenzene.
The completed reaction is shown in Figure 8.
(e)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The SN2 reaction is a nucleophilic substitution bimolecular reaction in which the addition of nucleophile and elimination of a leaving group takes place simultaneously. The rate of reaction depends upon both the substrate and reactant in the rate law equation.
Answer to Problem 10.59AP
The complete reaction is shown below.
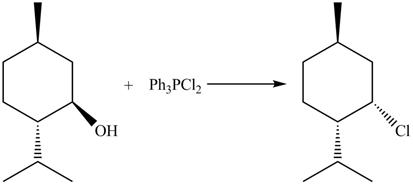
This is an SN2 reaction. Chloride group substitutes the hydroxide group and comes from the below the plane as the hydroxide group is above the plane.
Explanation of Solution
The given reaction is shown below.
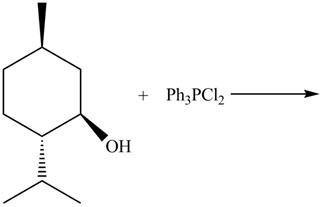
Figure 9
The complete reaction with the products is shown below.
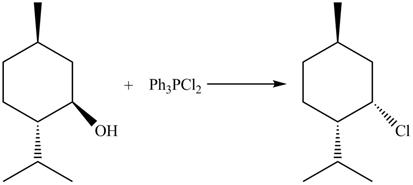
Figure 10
The reaction of alcohols with triphenylphosphine dichloride is a SN2 reaction. Chloride group substitutes the hydroxide group. The stereochemistry of the product is inverted in SN2 reaction. Therefore, chloride group goes below the plane.
The completed reaction is shown in Figure 10.
(f)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
An E2 reaction is a base-promoted β−elimination reaction. This reaction follows a concerted reaction mechanism. In this reaction, a β− proton is taken by the base and simultaneously leaving the group is eliminated. An
Answer to Problem 10.59AP
The complete reaction is shown below.
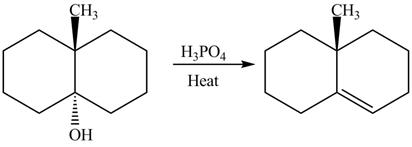
The reaction between an alcohol and acid with heating undergoes dehydration reaction to give alkene as a product.
Explanation of Solution
The given reaction is shown below.
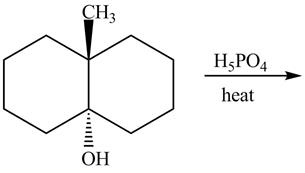
Figure 11
The complete reaction with the products is shown below.

Figure 12
The reaction of alcohols with acids and heat is an E2 reaction or dehydration reaction. The hydroxide is protonated at first and then the β− proton anti to the protonated hydroxide is taken up by the conjugate base of acid and simultaneously removes the protonated hydroxide group to give the alkene as a product.
The completed reaction is shown in Figure 12.
(g)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
An E2 reaction is a base-promoted β−elimination reaction. This reaction follows a concerted reaction mechanism. In this reaction, a β− proton is taken by the base and simultaneously leaving the group is eliminated. An alkene is obtained as a product of β−elimination reaction.
Answer to Problem 10.59AP
The complete reaction is shown below.
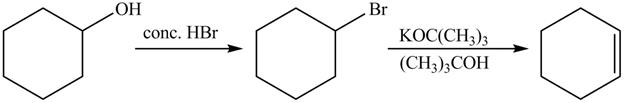
The first reaction is the nucleophilic substitution reaction of hydroxide group by the bromide ion. The second reaction is the elimination reaction in which strong base (CH3)3C−O− eliminates hydrogen bromide from the reactant to give alkene as a product.
Explanation of Solution
The given reaction is shown below.
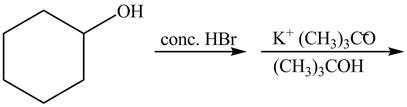
Figure 13
The complete reaction with the products is shown below.

Figure 14
The first step of the reaction is a SN2 reaction in which the bromide ion substitutes the protonated hydroxide group. In the second step, the strong and bulky base (CH3)3C−O− undergoes an E2 reaction and eliminates hydrogen bromide from the compound to give alkene product.
The completed reaction is shown in Figure 14.
(h)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
An E2 reaction is a base-promoted β−elimination reaction. This reaction follows a concerted reaction mechanism. In this reaction, a β- proton is taken by the base and simultaneously leaving the group is eliminated. An alkene is obtained as a product of β−elimination reaction.
Answer to Problem 10.59AP
The complete reaction is shown below.
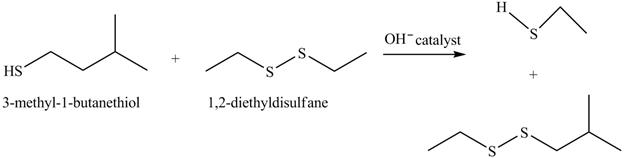
The acid-base reaction between the thiol group and hydroxide ion takes place first to give sulfide ion. The sulfide ion then reacts with diethyl sulfane to give a mixture of thiol and disulfide.
Explanation of Solution
The given reaction is shown below.
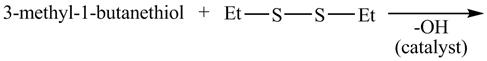
Figure 15
The complete reaction with the products is shown below.
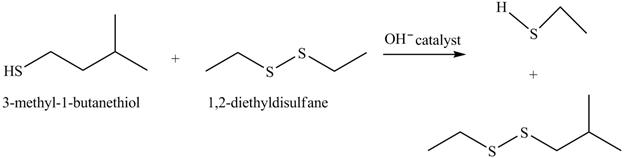
Figure 16
The hydroxide ion acts as a base and takes away the hydrogen of the thiol group of 3-methyl-1-butanethiol to give the sulfide ion. This ion then reacts with the diethyl sulfane to give a mixture of thiol and sulfane. The sulfide ion undergoes substitution reaction with the disulfides attacking at the one of the sulfur to give disulfide and thiol.
The completed reaction is shown in Figure 16.
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
- Please help with the curved arrow mechanism of this reaction, thank youarrow_forwardConcentration (mg/l) Peak Area 0 158 10 10241 20 18425 30 26457 40 37125 50 44256 60 56124 Question: Determine the regression equation (a and b coefficients) from first principlesarrow_forwardConcentration (mg/l) Peak Area 0 158 10 10241 20 18425 30 26457 40 37125 50 44256 60 56124 You have been asked to determine the concentration of citral in a highly valued magnolia essential oil. QUESTION: Calculate the concentration of citral in your highly valued magnolia essential oil which returns a peak area of 41658arrow_forward
- Need help with these problems...if you can please help me understand problems E & F.arrow_forwardPlease help me solve these problems. Thank you in advance.arrow_forwardPredict the products of this organic reaction: O N IN A N + H2O + HCI ? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. 田 C + Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- 6. For each of the following, fill in the synthesis arrows with reagents and show the intermediates. You DO NOT need to use the same number of arrows that are shown (you may use more or less), but the product must be formed from the reactant. Then write the mechanism of one step in the synthesis (you can choose which step to write the mechanism for), including all reagents required, clearly labeling the nucleophile and electrophile for each step, and using curved arrows to show the steps in the mechanism. a. b. OHarrow_forwardDraw the productsarrow_forwardDraw the correct productsarrow_forward
- E Organic Chemistry Maxwell Draw the correct products, in either order, for the ozonolysis reaction: 1) O3, CH2Cl2, -78 °C Product 1 + Product 2 2) Zn, HOAc Draw product 1. Select Draw Templates More C H O presented by M Draw product 2. Erase Select Draw Templates M / # # carrow_forward✓ edict the products of this organic reaction: ---- ။ A CH3–C−NH–CH2–C−CH3 + KOH ? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibiliarrow_forwardPredict the product of this organic reaction: A HO-C-CH3 + CH3NH2 P+ H2O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. marrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





