
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.81P
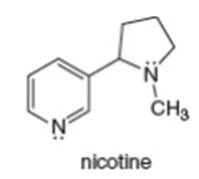
a. What is the hybridization of each N atom in nicotine?
b. What is the geometry around each N atom?
c. In what type of orbital does the lone pair on each N atom reside?
d. Draw a constitutional isomer of nicotine.
e. Draw a resonance structure of nicotine.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Given the major organic product(s) of each of the following reactions. If none is predicted, write "N.R."
answer]
a.
CHỊCH, CHẤT
AIC13
H
b.
0
Cl₂
HC-
NHOCH3
FeCl3
Give the major organic product(s) for each of the following reactions or sequences of reactions. Show
all relevant stereochemistry [2 ONLY].
A
-CH2COOH
1. LIAIH THF, heat
2 HO
B.
C.
CH₂Br
Br
1. NaCN, acetone
2 H₂O, heat
1. Mg ether
3 HO
Z CO₂
What is the order of increasing acidity for the following compounds? (least to most) [2 ONLY]
A.
COOH
COOH
COOH
COOH
6666
a.
IV, I, III, II
b.
III, II, I, IV
с.
II, III, I, IV
d. III, II, IV, I
slingoros
CH3
IV
woled noise biz
Chapter 1 Solutions
Organic Chemistry
Ch. 1 - While the most common isotope of nitrogen has a...Ch. 1 - Label each bond in the following compounds as...Ch. 1 - How many covalent bonds are predicted for each...Ch. 1 - Draw a valid Lewis structure for each species. a....Ch. 1 - Draw an acceptable Lewis structure for each...Ch. 1 - Prob. 1.6PCh. 1 - Draw a Lewis structure for each ion. a. CH3Ob....Ch. 1 - Draw Lewis structures for each molecular formula....Ch. 1 - Prob. 1.9PCh. 1 - Prob. 1.10P
Ch. 1 - Prob. 1.11PCh. 1 - Prob. 1.12PCh. 1 - Draw a second resonance structure for each...Ch. 1 - Prob. 1.14PCh. 1 - Draw a second resonance structure for nitrous...Ch. 1 - Prob. 1.16PCh. 1 - Prob. 1.17PCh. 1 - Prob. 1.18PCh. 1 - Prob. 1.19PCh. 1 - Prob. 1.20PCh. 1 - Simplify each condensed structure by using...Ch. 1 - Prob. 1.22PCh. 1 - Prob. 1.23PCh. 1 - Convert each skeletal structure to a complete...Ch. 1 - Draw in all hydrogens and lone pairs on the...Ch. 1 - Prob. 1.26PCh. 1 - What orbitals are used to form each of the CC, and...Ch. 1 - What orbitals are used to form each bond in the...Ch. 1 - Determine the hybridization around the highlighted...Ch. 1 - Classify each bond in the following molecules as ...Ch. 1 - Prob. 1.31PCh. 1 - Rank the following atoms in order of increasing...Ch. 1 - Prob. 1.33PCh. 1 - Prob. 1.34PCh. 1 - Provide the following information about...Ch. 1 - Use the ball-and-stick model to answer each...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Two radioactive isotopes of iodine used for the...Ch. 1 - Prob. 1.40PCh. 1 - Assign formal charges to each carbon atom in the...Ch. 1 - Assign formal charges to each N and O atom in the...Ch. 1 - Draw one valid Lewis structure for each compound....Ch. 1 - Prob. 1.44PCh. 1 - Prob. 1.45PCh. 1 - Prob. 1.46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 1.49PCh. 1 - Prob. 1.50PCh. 1 - Prob. 1.51PCh. 1 - Prob. 1.52PCh. 1 - Prob. 1.53PCh. 1 - Prob. 1.54PCh. 1 - Draw all reasonable resonance structures for each...Ch. 1 - Prob. 1.56PCh. 1 - Rank the resonance structures in each group in...Ch. 1 - 1.56 Consider the compounds and ions with curved...Ch. 1 - 1.57 Predict all bond angles in each...Ch. 1 - Predict the geometry around each indicated atom....Ch. 1 - Prob. 1.61PCh. 1 - Prob. 1.62PCh. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - Prob. 1.64PCh. 1 - Prob. 1.65PCh. 1 - Prob. 1.66PCh. 1 - Prob. 1.67PCh. 1 - Each of the following condensed or skeletal...Ch. 1 - Prob. 1.69PCh. 1 - Prob. 1.70PCh. 1 - Prob. 1.71PCh. 1 - Prob. 1.72PCh. 1 - Prob. 1.73PCh. 1 - Prob. 1.74PCh. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Label the polar bonds in each molecule. Indicate...Ch. 1 - Answer the following questions about acetonitrile...Ch. 1 - Prob. 1.79PCh. 1 - The principles of this chapter can be applied to...Ch. 1 -
a. What is the hybridization of each N atom in...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 1.85PCh. 1 - Prob. 1.86PCh. 1 - Prob. 1.87PCh. 1 - Prob. 1.88PCh. 1 - Prob. 1.89PCh. 1 - Prob. 1.90P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
11. In the early 1800s, French naturalist Jean Baptiste Lamarck suggested that the best explanation for the rel...
Campbell Biology: Concepts & Connections (9th Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- With this, please answer the following questions: 1.) draw the structure of the electrophilic intermediate in this reaction. 2.) what is the role of the AlCl3 in the reaction 3.) write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and include all intermediate structuresarrow_forwardConsider the data below to answer the following questions. Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. Unfortunately, when a cyanohydrin is treated with aqueous base the original carbonyl compound is isolated. HO H HCEN H-3- HO' NaOH HO cyanohychin a. a nucleophilic substitution b. an electrophilic addition C10 OH CH-COOH A. The reaction of an aldehyde with hydrogen cyanide is an example of + NaCN + H₂O reaction. H- C. an electrophilic substitution d. a nucleophilic addition B. Identify the electrophile in the reaction of benzaldehyde with hydrogen cyanide.arrow_forwardRefer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A. Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- Give the major organic product(s) of each of the following reactions or sequences of reactions. Show all relevant stereochemistry.[4 only] CH3 A. B. HNO H₂Pt H₂SO4 hano NaN 1. LIAH ether Br 4 2 H₂O C. D. E. CH3CH2-CH2CH3 + HCl Br NH₂ CH3 ON CH-CH3 Br HNOZ CUCI 11,504 HC) 1. HNO H SO NH₂ 2 UMarrow_forwardConsider the Grignard reaction below to answer the following questions. A Mgar 1. ether + MyC CH3 2H3O C B a. The electrophile in this reaction is: b. The nucleophile in this reaction is: c. The alcohol product can be classified as a: a. 1° alcohol b. 2° alcohol C. 3° alcohol d. 4° alcohol HO CH3 CHarrow_forwardGive the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry A. CH₂OH PCC CH2Cl2 HOO B. H KCN HCN of b C. 1. CH,MgBr, ether 2 HO* D. Choose the BEST reagent for carrying out each of the following conversions. CO₂CH3 CO₂CH3 OH CO₂H сон ن نے a. LiAlH4, ether at abinayo iss c b. NaBH4, ethanol C. CrO3, pyridine d. H₂/Pd d notsiolarrow_forward
- Choose the best reagent for carrying out the following reactions from the list below. Place the letter of the reagent(s) in the box over the reaction arrow. Use only one letter per box. OH OH CH CH CH3 CHS CH3 f OH OCH 3 H A. NaH, then CHI B. C. m-ClC6H4COзH D. E. warm H2SO4/H₂O F. G. H₂/Pd H. I. Cl₂, H₂O J. NaOCH3, CH3OH CH3MgBr in ether, then H3O+ Hg(O2CCF3)2, CH3OH PCC, CH2Cl2 LiAlH4 in ether, then H3O+arrow_forwardWhat is the product of the reaction of 2,4-pentanedione with phenylhydrazine?arrow_forwardIn the reaction of naphthalene with CrO3 in acetic acid. Indicate whether a different product is obtained if carried out at 25°C or with heating (A).arrow_forward
- QUESTION: Fill in the answers in the empty green boxes 1. Step 2 2. Step 3 3. Step 4 (SUM) 4. Step 5 (df) (GIVEN) 5. Determine S y/x value *The data values have been provided in the worksheet attached in the first image*arrow_forwardIf the symbol A is placed in a reaction, at what temperature does it take place?arrow_forwardBy malonic or acetylacetic synthesis, synthesize 3-methyl-4-oxopentanoic acid (indicate the formulas of the compounds).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY