
Concept explainers
Stalevo is the trade name for a medication used fssor Parkinson’s disease, which contains L-dopa, carbidopa, and entacapone.
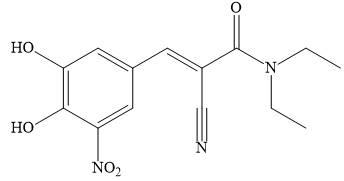
a. Draw a Lewis structure for entacapone.
b. Which
c. Which
d. Which
e. Which
f. Use curved arrows to draw a resonance structure that is an equal contributor to the resonance hybrid.
g. Use curved arrows to draw a resonance structure that is a minor contributor to the resonance hybrid.
(a)
Interpretation: A Lewis structure for entacapone is to be drawn.
Concept introduction: One should follow the given steps to draw the Lewis structure of a given skeleton structure. The first step is placing a
Answer to Problem 1.82P
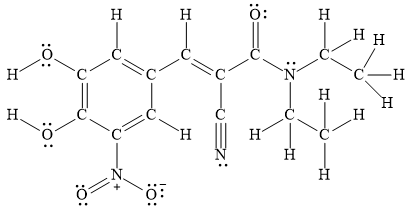
A Lewis structure for entacapone is,
Explanation of Solution
The given structure of entacapone is in skeleton form.
One should follow the given steps to draw the Lewis structure of a given skeleton structure. The first step is placing a
Thus, a Lewis structure for entacapone is,

Figure 1
A Lewis structure for entacapone is shown in Figure 1.
(b)
Interpretation: The longest
Concept introduction: Bond length is inversely proportional to the number of electrons present between two nuclei. This means as the number of electrons present between two nuclei increases bond length becomes shorter and stronger.
Answer to Problem 1.82P
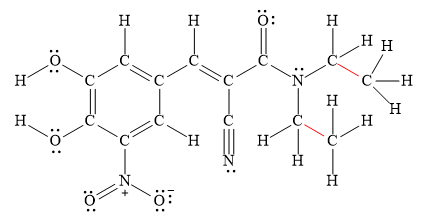
The
Explanation of Solution
Bond length is inversely proportional to the number of electrons present between two nuclei. This means as the number of electrons present between two nuclei increases bond length becomes shorter and stronger.
Therefore, triple bonds are stronger and shorter than double bonds, which are stronger than single bonds.
The longest

Figure 2
Thus, the
The
(c)
Interpretation: The shortest
Concept introduction: Bond length is inversely proportional to the number of electrons present between two nuclei. This means as the number of electrons present between two nuclei increases bond length becomes shorter and stronger.
Answer to Problem 1.82P
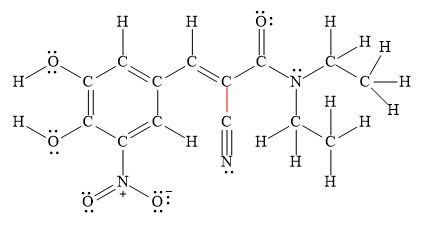
The shortest
Explanation of Solution
Bond length is inversely proportional to the number of electrons present between two nuclei. This means as the number of electrons present between two nuclei increases bond length becomes shorter and stronger.
Therefore, triple bonds are stronger and shorter than double bonds, which are stronger than single bonds.
The shortest

Figure 3
Thus, the shortest
The shortest
(d)
Interpretation: The longest
Concept introduction: Bond length is inversely proportional to the number of electrons present between two nuclei. This means as the number of electrons present between two nuclei increases bond length becomes shorter and stronger.
Answer to Problem 1.82P
The longest
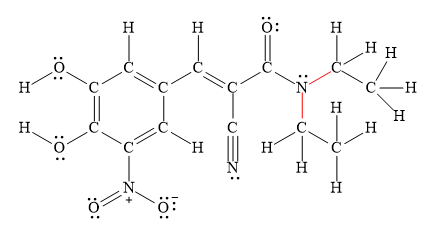
Explanation of Solution
Bond length is inversely proportional to the number of electrons present between two nuclei. This means as the number of electrons present between two nuclei increases bond length becomes shorter and stronger.
Therefore, triple bonds are stronger and shorter than double bonds, which are stronger than single bonds.
The longest

Figure 4
Thus, the longest
The longest
(e)
Interpretation: The shortest
Concept introduction: Bond length is inversely proportional to the number of electrons present between two nuclei. This means as the number of electrons present between two nuclei increases bond length becomes shorter and stronger.
Answer to Problem 1.82P
The shortest
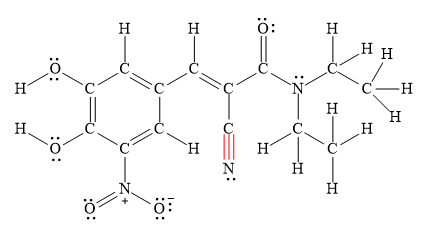
Explanation of Solution
Bond length is inversely proportional to the number of electrons present between two nuclei. This means as the number of electrons present between two nuclei increases bond length becomes shorter and stronger.
Therefore, triple bonds are stronger and shorter than double bonds, which are stronger than single bonds.
The shortest

Figure 5
Thus, the shortest
The shortest
(f)
Interpretation: A resonance structure that is an equal contributor to the resonance hybrid in entacapone is to be drawn.
Concept introduction: The delocalization of lone pair or free electrons from one atom to another is known as resonance. The stability of compound depends upon the number of resonating structures. More the resonating structures of compound more will its stability.
Answer to Problem 1.82P
A resonance structure that is an equal contributor to the resonance hybrid in entacapone is drawn below.
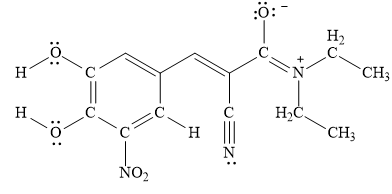
Explanation of Solution
The delocalization of lone pair or free electrons from one atom to another is known as resonance. The stability of compound depends upon the number of resonating structures. More the resonating structures of compound more will its stability.
A resonance structure that is an equal contributor to the resonance hybrid in entacapone is drawn below.
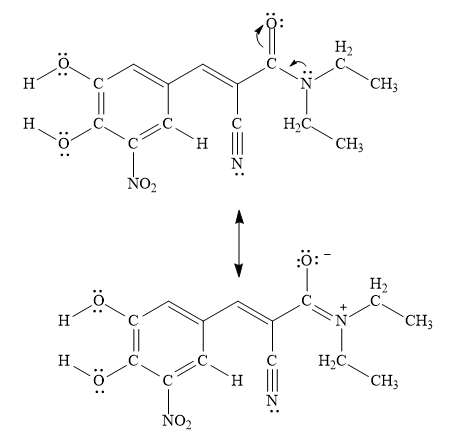
Figure 6
A resonance structure that is an equal contributor to the resonance hybrid in entacapone is drawn in Figure 6.
(g)
Interpretation: A resonance structure that is minor contributor to the resonance hybrid in entacapone is to be drawn.
Concept introduction: The delocalization of lone pair or free electrons from one atom to another is known as resonance. The stability of compound depends upon the number of resonating structures. More the resonating structures of compound more will its stability.
Answer to Problem 1.82P
A resonance structure that is minor contributor to the resonance hybrid in entacapone is drawn below.
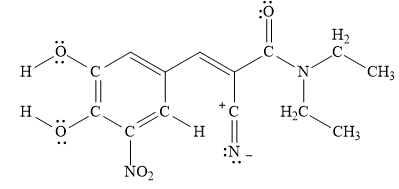
Explanation of Solution
The delocalization of lone pair or free electrons from one atom to another is known as resonance. The stability of compound depends upon the number of resonating structures. More the resonating structures of compound more will its stability.
A resonance structure that is minor contributor to the resonance hybrid in entacapone is drawn below.
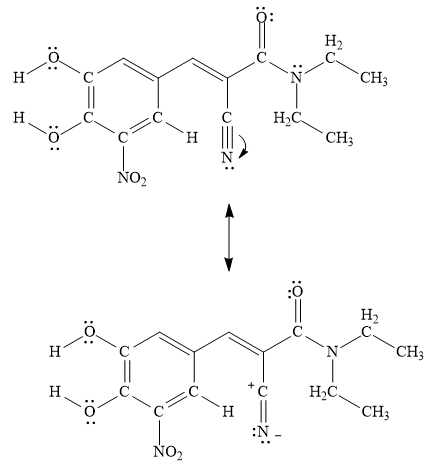
Figure 7
A resonance structure that is minor contributor to the resonance hybrid in entacapone is drawn in Figure 7.
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





