
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.42P
![]() Assign formal charges to each N and O atom in the given molecules. All lone pairs have been drawn in.
Assign formal charges to each N and O atom in the given molecules. All lone pairs have been drawn in.
a.
![]()
b.
![]()
C.
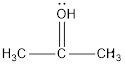
d.
![]()
e.
![]()
f.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the relationship between the limiting reactant and theoretical yield of CO2?
From your calculations, which reaction experiment had closest to stoichiometric quantities? How many moles of NaHCO3 and HC2H3O2 were present in this reaction?
18. Arrange the following carbocations in order of decreasing stability.
1
2
A 3124
B 4213 C 2431
D 1234
E 2134
SPL
3
4
Chapter 1 Solutions
Organic Chemistry
Ch. 1 - While the most common isotope of nitrogen has a...Ch. 1 - Label each bond in the following compounds as...Ch. 1 - How many covalent bonds are predicted for each...Ch. 1 - Draw a valid Lewis structure for each species. a....Ch. 1 - Draw an acceptable Lewis structure for each...Ch. 1 - Prob. 1.6PCh. 1 - Draw a Lewis structure for each ion. a. CH3Ob....Ch. 1 - Draw Lewis structures for each molecular formula....Ch. 1 - Prob. 1.9PCh. 1 - Prob. 1.10P
Ch. 1 - Prob. 1.11PCh. 1 - Prob. 1.12PCh. 1 - Draw a second resonance structure for each...Ch. 1 - Prob. 1.14PCh. 1 - Draw a second resonance structure for nitrous...Ch. 1 - Prob. 1.16PCh. 1 - Prob. 1.17PCh. 1 - Prob. 1.18PCh. 1 - Prob. 1.19PCh. 1 - Prob. 1.20PCh. 1 - Simplify each condensed structure by using...Ch. 1 - Prob. 1.22PCh. 1 - Prob. 1.23PCh. 1 - Convert each skeletal structure to a complete...Ch. 1 - Draw in all hydrogens and lone pairs on the...Ch. 1 - Prob. 1.26PCh. 1 - What orbitals are used to form each of the CC, and...Ch. 1 - What orbitals are used to form each bond in the...Ch. 1 - Determine the hybridization around the highlighted...Ch. 1 - Classify each bond in the following molecules as ...Ch. 1 - Prob. 1.31PCh. 1 - Rank the following atoms in order of increasing...Ch. 1 - Prob. 1.33PCh. 1 - Prob. 1.34PCh. 1 - Provide the following information about...Ch. 1 - Use the ball-and-stick model to answer each...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Two radioactive isotopes of iodine used for the...Ch. 1 - Prob. 1.40PCh. 1 - Assign formal charges to each carbon atom in the...Ch. 1 - Assign formal charges to each N and O atom in the...Ch. 1 - Draw one valid Lewis structure for each compound....Ch. 1 - Prob. 1.44PCh. 1 - Prob. 1.45PCh. 1 - Prob. 1.46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 1.49PCh. 1 - Prob. 1.50PCh. 1 - Prob. 1.51PCh. 1 - Prob. 1.52PCh. 1 - Prob. 1.53PCh. 1 - Prob. 1.54PCh. 1 - Draw all reasonable resonance structures for each...Ch. 1 - Prob. 1.56PCh. 1 - Rank the resonance structures in each group in...Ch. 1 - 1.56 Consider the compounds and ions with curved...Ch. 1 - 1.57 Predict all bond angles in each...Ch. 1 - Predict the geometry around each indicated atom....Ch. 1 - Prob. 1.61PCh. 1 - Prob. 1.62PCh. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - Prob. 1.64PCh. 1 - Prob. 1.65PCh. 1 - Prob. 1.66PCh. 1 - Prob. 1.67PCh. 1 - Each of the following condensed or skeletal...Ch. 1 - Prob. 1.69PCh. 1 - Prob. 1.70PCh. 1 - Prob. 1.71PCh. 1 - Prob. 1.72PCh. 1 - Prob. 1.73PCh. 1 - Prob. 1.74PCh. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Label the polar bonds in each molecule. Indicate...Ch. 1 - Answer the following questions about acetonitrile...Ch. 1 - Prob. 1.79PCh. 1 - The principles of this chapter can be applied to...Ch. 1 -
a. What is the hybridization of each N atom in...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 1.85PCh. 1 - Prob. 1.86PCh. 1 - Prob. 1.87PCh. 1 - Prob. 1.88PCh. 1 - Prob. 1.89PCh. 1 - Prob. 1.90P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Acetic acid is added to DI water at an initial concentration of 10 -6 M (Ka=1.8x10-5) A. Using the "ICE" Method, what would the pH be at equilibrium? State assumptions and show your work. B. Using the simultaneous equations method, what would the pH be at equilibrium? Show your workarrow_forward1. Show that the change in entropy for a fixed amount of ideal gas held at a constant temperature undergoing a volume change is given by the simple equation AS = NkB In Hint: Start with the equation M dS = du + (Œ) dv - Ž (#) an, dU du+av-dN; j=1 Why doesn't the equation for the entropy of an ideal gas depend on the strength of the intermolecular forces for the gas?arrow_forward2. Make an ice cube at 1 bar pressure by freezing an amount of liquid water that is 2 cm x 2 cm x 2 cm in volume. The density of liquid water at 0 °C is 1.000 g cm³ and the density of ice at 0 °C is 0.915 g cm³. Note that this difference in density is the reason your water pipes burst if they freeze and why you shouldn't forget to take your bottle of pop out of the freezer if you put it in there to try and cool it down faster. A. What is the work of expansion upon freezing? B. Is work done on the system or by the system?arrow_forward
- I have a excitation/emission spectra of a quinine standard solution here, and I'm having trouble interpreting it. the red line is emission the blue line is excitation. i'm having trouble interpreting properly. just want to know if there is any evidence of raman or rayleigh peaks in the spectra.arrow_forwardGive the major product of the following reaction. excess 1. OH, H₂O 1.OH H CH3CH2CH21 H 2. A.-H₂O Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default.arrow_forward2. Use Hess's law to calculate the AH (in kJ) for: rxn CIF(g) + F2(g) → CIF 3 (1) using the following information: 2CIF(g) + O2(g) → Cl₂O(g) + OF 2(g) AH = 167.5 kJ ΔΗ 2F2 (g) + O2(g) → 2 OF 2(g) 2C1F3 (1) + 202(g) → Cl₂O(g) + 3 OF 2(g) о = = -43.5 kJ AH = 394.1kJarrow_forward
- The combustion of 28.8 g of NH3 consumes exactly _____ g of O2. 4 NH3 + 7 O2 ----> 4 NO2 + 6 H2Oarrow_forwardWhat is the molecular formula of the bond-line structure shown below OH HO ○ C14H12O2 ○ C16H14O2 ○ C16H12O2 O C14H14O2arrow_forwardCheck all molecules that are acids on the list below. H2CO3 HC2H3O2 C6H5NH2 HNO3 NH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY