
Concept explainers
(a)
Draw the
(a)
Answer to Problem 173RP
The
Explanation of Solution
Draw the
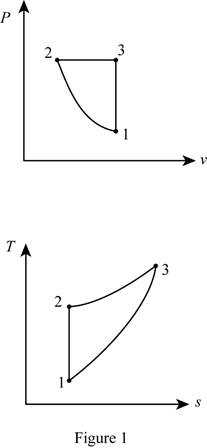
Thus, the
(b)
The expression for the back work ratio as a function of k and r.
(b)
Answer to Problem 173RP
The expression for the back work ratio as a function of k and r is
Explanation of Solution
Find the work of compression using the first law for process 1-2.
Here, heat interaction during the process 1-2 is
Write the expression of expansion work.
Here, gas constant is R, specific volume at state 2 and 3 is
Write the expression of back work ratio using the equations (I) and (II).
Here, temperature at state 1, 2, and 3 are
Conclusion:
Process 1-2: Isentropic
Calculate the ratio of
Here, pressure at state 1 and 2 is
Process 2-3: Constant pressure
Calculate the expression for
Here, volume at state 1 and 2 is
Process 3-1: Constant volume
Calculate the expression for
Substitute
Thus, the expression for the back work ratio as a function of k and r is
(c)
The expression for the cycle thermal efficiency as a function of k and r.
(c)
Answer to Problem 173RP
The expression for the cycle thermal efficiency as a function of k and r is
Explanation of Solution
Express out the heat addition and heat rejection in the process using first law to the closed system for processes 2-3 and 3-1.
Here, constant pressure specific heat is
Express the cycle thermal efficiency.
Conclusion:
Substitute
Substitute
Thus, the expression for the cycle thermal efficiency as a function of k and r is
(d)
The value of the back work ratio and thermal efficiency as r goes to unity.
(d)
Answer to Problem 173RP
The value of the back work ratio and thermal efficiency as r goes to unity is
Explanation of Solution
Recall the expression of back work ratio and apply the limits as r goes to infinity.
Thus, the value of the back work ratio as r goes to unity is
Recall the expression of cycle thermal efficiency and apply the limits as r goes to infinity.
Thus, the value of the thermal efficiency as r goes to unity is
From the results of cycle thermal efficiency and back work ratio values of 0 and 1, it shows that no expansion and net work can be done whether you add heat to the system when there is no compression
Want to see more full solutions like this?
Chapter 9 Solutions
Thermodynamics: An Engineering Approach
- How to solve this?arrow_forwardA start-up company wants to convert an ICE vehicle into an electric vehicle with the following specification. Power: 250 (HP) horsepower, (note: 1HP = 745 W) Range: 300-miles Fuel economy: 33.5 kilometers per gallon of gasoline. Efficiency of the ICE: 25% Energy Conversion: One gallon of gasoline at 100% efficiency is equal to 33.5 kWh/gallon). a)Calculate the EV consumption rate as Wh/km and find the total energy of the battery pack in KWh to replace the internal combustion engine. b)Design an 8-module battery pack for this full electric vehicle without compromising its range and performance (power). Use commercially available cylindrical cells lithium cell with 20Ah capacity and 3.125 V average voltage. Cell dimensions are 5cm diameter and 10 cm height. The electric motor requires 250 V input that will be provided directly from the battery pack, Report the configuration of each module in…arrow_forward"11-17 The shaft shown in Figure P11-3 was designed in Problem 10-17. For the data in the row(s) assigned from Table P11-1, and the corresponding diameter of shaft found in Problem 10-17, design suitable bearings to support the load for at least 1E8 cycles at 1800 rpm. State all assumptions. (a) Using hydrodynamically lubricated bronze sleeve bearings with Ox = 15, 11d=0.75, and a clearance ratio of 0.001. ✓ ✓ cast-iron roller FIGURE P11-3 Shaft Design for Problems 11-17 b gear key assume bearings act as simple supports 11-19 The shaft shown in Figure P11-4 was designed in Problem 10-19. For the data in the row(s) assigned from Table P11-1, and the corresponding diameter of shaft found in Problem 10-19, design suitable bearings to support the load for at least 5E8 cycles at 1200 rpm. State all assumptions. (a) Using hydrodynamically lubricated bronze sleeve bearings with Oy = 40, 1/d=0.80, and a clearance ratio of 0.002 5. gear gear key FIGURE P11-4 Shaft Design for Problems 11-19 and…arrow_forward
- For the frame below calculate the bending moment at point R. Take P=40 and note that this value is used for both the loads and the lengths of the members of the frame. 2.5P- A Q B R С 45 degrees ✗ ✗ P i 19 Кур -2P- 4PRN -P- -arrow_forwardCalculate the bending moment at the point D on the beam below. Take F=79 and remember that this quantity is to be used to calculate both forces and lengths. 15F 30F A сarrow_forwardShow work on how to obtain P2 and T2. If using any table, please refer to it. If applying interpolation method, please show the work.arrow_forwardcast-iron roller FIGURE P11-3 Shaft Design for Problems 11-17 Chapter 11 BEARINGS AND LUBRICATION 677 gear key P assume bearings act as simple supports 11-18 Problem 7-18 determined the half-width of the contact patch for a 1.575-in-dia steel cylinder, 9.843 in long, rolled against a flat aluminum plate with 900 lb of force to be 0.0064 in. If the cylinder rolls at 800 rpm, determine its lubrication condition with ISO VG 1000 oil at 200°F. R₁ = 64 μin (cylinder); R₁ = 32 μin (plate). 11-19 The shaft shown in Figure P11-4 was designed in Problem 10-19. For the data in the row(s) assigned from Table P11-1, and the corresponding diameter of shaft found in Problem 10-19, design suitable bearings to support the load for at least 5E8 cycles at 1200 rpm. State all assumptions. (a) (b) Using hydrodynamically lubricated bronze sleeve bearings with ON = 40, 1/ d=0.80, and a clearance ratio of 0.002 5. Using deep-groove ball bearings for a 10% failure rate. *11-20 Problem 7-20 determined the…arrow_forwardCalculate the shear force at the point D on the beam below. Take F=19 and remember that this quantity is to be used to calculate both forces and lengths. 15F A сarrow_forward"II-1 The shaft shown in Figure P11-I was designed in Problem 10-1. For the data in the row(s) assigned from Table P11-1, and the corresponding diameter of shaft found in Problem 10-1, design suitable bearings to support the load for at least 7E7 cycles at 1500 rpm. State all assumptions. (a) Using hydrodynamically lubricated bronze sleeve bearings with Ox = 20, 1/d=1.25, and a clearance ratio of 0.001 5. assume bearings act as simple supports FIGURE P11-1 Shaft Design for Problem 11-1 11-2 The shaft shown in Figure P11-2 was designed in Problem 10-2. For the data in the row(s) assigned from Table P11-1, and the corresponding diameter of shaft found in Problem 10-2, design suitable bearings to support the load for at least 3E8 cycles at 2.500 rpm. State all assumptions. (a) Using hydrodynamically lubricated bronze sleeve bearings with ON=30, 1/d=1.0, and a clearance ratio of 0.002. FIGURE P11-2 Shaft Design for Problem 11-2 Table P11-1 Data for Problems assume bearings act as simple…arrow_forwardFor the frame below, calculate the shear force at point Q. Take P=13 and note that this value is used for both the loads and the lengths of the members of the frame. 1 A Q ✗ 19 KBP 2.5P- B R C 45 degrees ✗ 1 .2P- 4PhN -P→arrow_forwardCalculate the Bending Moment at point D in the frame below. Leave your answer in Nm (newton-metres) J J A 2m 2m <2m х D 不 1m X E 5m 325 Nm 4x 400N/marrow_forwardIn the beam below, calculate the shear force at point A. Take L=78 and remember that both the loads and the dimensions are expressed in terms of L. 143 1 DX A - Li 4 LhN 14LRN/m Х B 22 3 L.arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_iosRecommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY