
The specific impulse of the jet engine.
Answer to Problem 170RP
The specific impulse of the jet engine is
Explanation of Solution
Draw the
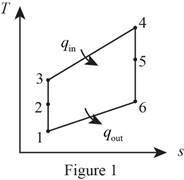
Consider that the aircraft is stationary, and the velocity of air moving towards the aircraft is
Diffuser :
Write the expression for the energy balance equation for the diffuser.
Here, the rate of energy entering the system is
Write the temperature and pressure relation for the process 1-2.
Here, the specific heat ratio of air is k, pressure at state 1 is
Compressor:
Write the pressure relation using the pressure ratio for the process 2-3.
Here, the pressure ratio is
Write the temperature and pressure relation for the process 2-3.
Here, temperate at state 3 is
Turbine:
Write the temperature relation for the compressor and turbine.
Here, the specific heat at constant pressure is
, temperature at state 4 is
Nozzle:
Write the temperature and pressure relation for the isentropic process 4-6.
Here, pressure at state 6 is
Write the energy balance equation for the nozzle.
Write the expression to calculate the specific impulse of the jet engine.
Here, the thrust force produced by engine is
Conclusion.
From Table A-1E, “Molar mass, gas constant, and critical-point properties”, obtain the
value of gas constant
From Table A-2Ea, “Ideal-gas specific heats of various common gases”, obtain the following values for air at room temperature.
The rate of change in the energy of the system
Substitute
Here, inlet velocity is
Substitute 0 for
Substitute 10psia for
Substitute 9 for
Substitute 609.8 R for
Substitute
Substitute
The rate of change in the energy of the system
Substitute
Here, velocity at stat 5 is
Since,
Substitute
Substitute
Thus, the specific impulse of the jet engine is
Want to see more full solutions like this?
Chapter 9 Solutions
Thermodynamics: An Engineering Approach
- USE MATHLAB WITH CODES Estimate the damping ratio, stiffness, natural frequency, and mass of the SDOF system. Please use a MATHLAB with CODES and no negative damping ratio. Data Set 1:Time(s) Data Set 1:top1(g) Data Set 1:bottom(g)0 0.002593181 0.007262860.01 0.011367107528507709 -0.0015110660.02 0.007467585 -0.0058980290.029999999999999999 0.004542943 0.0028758970.040000000000000001 0.018678712689042091 -0.0019985060.050000000000000003 0.004542943 0.0009261360.059999999999999998 0.014779189431130886 -0.0068729090.070000000000000007 0.004055502 -0.0088226710.080000000000000002 0.008442465 -0.0015110660.089999999999999997 0.011854547366917134 -0.0039482670.10000000000000001 0.007467585 0.0058005390.11 0.004055502 0.0043382180.12 0.010392226334810257 0.0019010160.13 0.010392226334810257 -0.001998506% 0.14000000000000001 0.016728950301647186 0.0048256580.14999999999999999 0.007955025…arrow_forwardProvide an example of at least five features produced by a certain machining process (for example, a keyway to accommodate a key iarrow_forwardHow to draw a gam from the data of the subject's readings three times and difficulties in drawing a gam Material Name: Machinery Theory I'm a vehicle engineering student. Please describe details about gam in addition the law gam: 1-tangent cam with reciprocating roller follower. 2-circular arc cam with flat-faced follower.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





