
(a)
Interpretation:
The member that rapidly undergoes
Concept Introduction:
The
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
(a)
Answer to Problem 9.25P
The member that rapidly undergoes
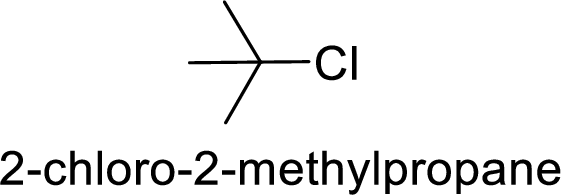
Explanation of Solution
Given:
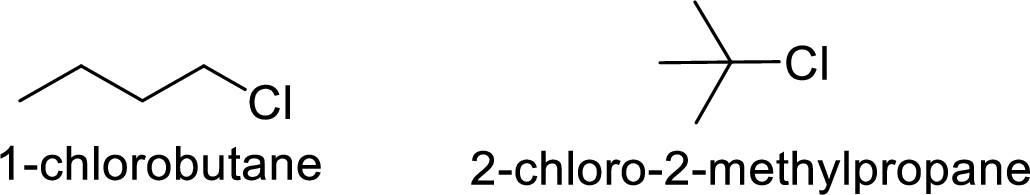
In
2-chloro-2 methyl propane gives more stable carbocation than 1-chlorobutane.
Hence, the member that rapidly undergoes
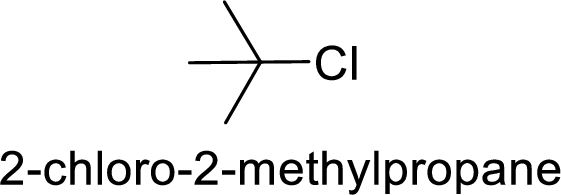
(b)
Interpretation:
The member that rapidly undergoes
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
(b)
Answer to Problem 9.25P
The member that rapidly undergoes
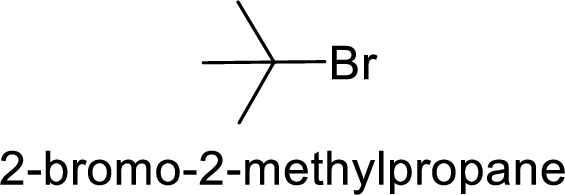
Explanation of Solution
Given:
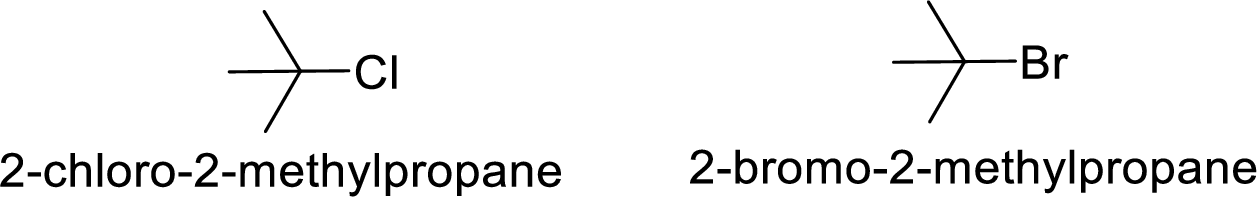
Bromine is better leaving group than chlorine.
Hence, the member that rapidly undergoes
(c)
Interpretation:
The member that rapidly undergoes
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
(c)
Answer to Problem 9.25P
The member that rapidly undergoes
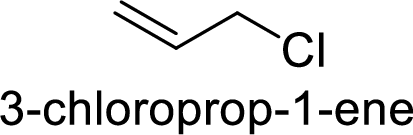
Explanation of Solution
Given:

In
Allyl cation is more stable than primary cation.
Hence, the member that rapidly undergoes
(d)
Interpretation:
The member that rapidly undergoes
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
(d)
Answer to Problem 9.25P
The member that rapidly undergoes
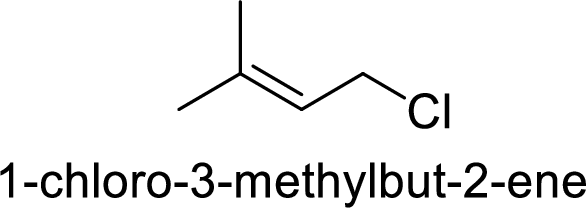
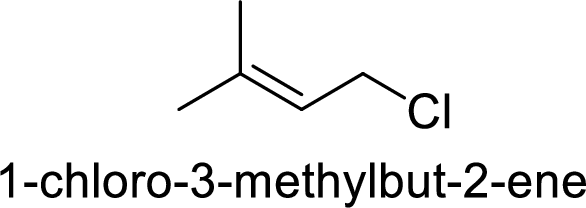
Explanation of Solution
Given:

In
Substuted allyllic cation is more stable than allylic cation.
Hence, the member that rapidly undergoes
(e)
Interpretation:
The member that rapidly undergoes
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
(e)
Answer to Problem 9.25P
The member that rapidly undergoes
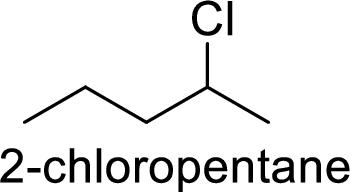
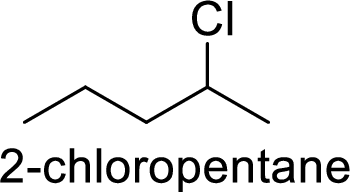
Explanation of Solution
Given:

In
2-chloropentane gives more stable carbocation than 1-chloropentane.
Hence, the member that rapidly undergoes
(f)
Interpretation:
The member that rapidly undergoes
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
(f)
Answer to Problem 9.25P
The member that rapidly undergoes
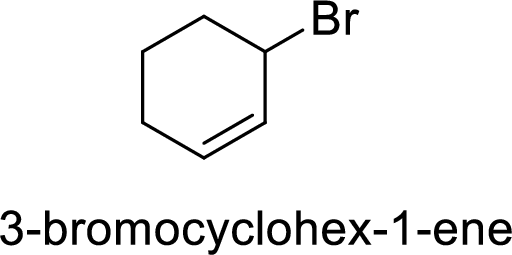
Explanation of Solution
Given:
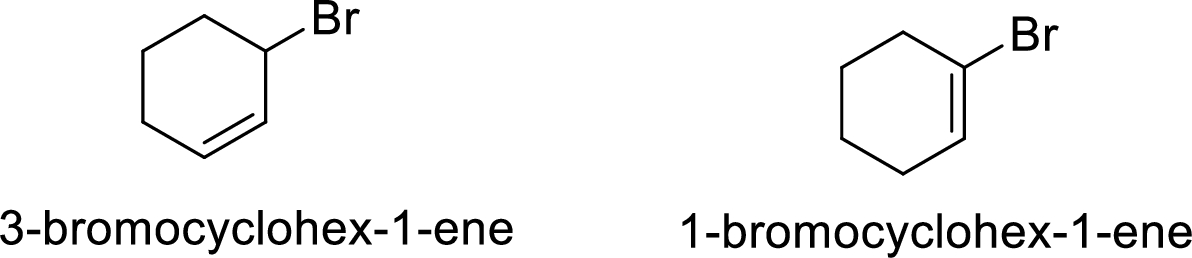
In
Allylic cation is more stable than vinyl cation.
Hence, the member that rapidly undergoes
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry
- Help me solve this problem. Thank you in advance.arrow_forward22.7 Predict the monoalkylated products of the following reactions with benzene. (a) AlCl3 Ya (b) AlCl3 (c) H3PO4 (d) 22.8 Think-Pair-Share AICI3 The reaction below is a common electrophilic aromatic substitution. SO3 H₂SO4 SO₂H (a) Draw the reaction mechanism for this reaction using HSO,+ as the electrophile. (b) Sketch the reaction coordinate diagram, where the product is lower in energy than the starting reactant. (c) Which step in the reaction mechanism is highest in energy? Explain. (d) Which of the following reaction conditions could be used in an electrophilic aro- matic substitution with benzene to provide substituted phenyl derivatives? (i) AICI3 HNO3 H₂SO4 K2Cr2O7 (iii) H₂SO4 (iv) H₂PO₁arrow_forwardIs an acid-base reaction the only type of reaction that would cause leavening products to rise?arrow_forward
- Help me understand this! Thank you in advance.arrow_forward22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forwardV Consider this step in a radical reaction: Br: ? What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ⚫ionization termination initialization neutralization none of the abc Explanation Check 80 Ο F3 F1 F2 2 F4 01 % do5 $ 94 #3 X 5 C MacBook Air 25 F5 F6 66 ©2025 ˇ F7 29 & 7 8arrow_forward
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


