
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN: 9781133104261
Author: Raymond A. Serway, John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 55P
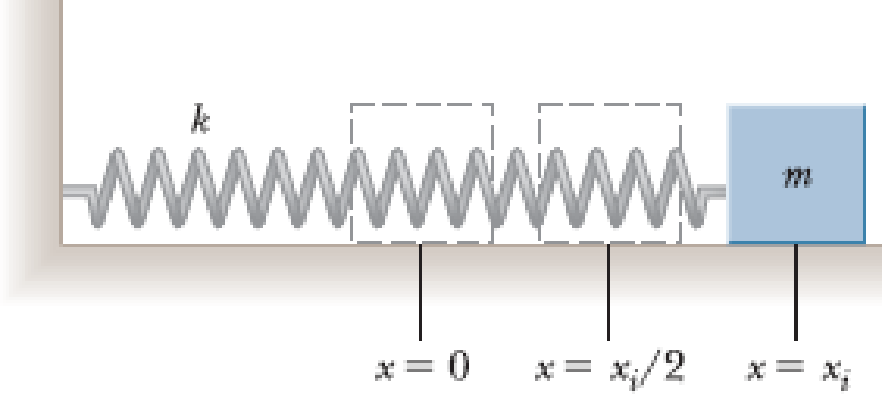
A horizontal spring attached to a wall has a force constant of k = 850 N/m. A block of mass m = 1.00 kg is attached to the spring and rests on a frictionless, horizontal surface as in Figure P7.55. (a) The block is pulled to a position xi = 6.00 cm from equilibrium and released. Find the elastic potential energy stored in the spring when the block is 6.00 cm from equilibrium and when the block passes through equilibrium. (b) Find the speed of the block as it passes through the equilibrium point. (c) What is the speed of the block when it is at a position xi/2 = 3.00 cm? (d) Why isn’t the answer to part (c) half the answer to part (b)?
Figure P7.55
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Imagine you are out for a stroll on a sunny day when you encounter a lake. Unpolarized light from the sun is reflected off the lake into your eyes. However, you notice when you put on your vertically polarized sunglasses, the light reflected off the lake no longer reaches your eyes. What is the angle between the unpolarized light and the surface of the water, in degrees, measured from the horizontal? You may assume the index of refraction of air is nair=1 and the index of refraction of water is nwater=1.33 . Round your answer to three significant figures. Just enter the number, nothing else.
Deduce what overvoltage is like in reversible electrodes.
pls help on these
Chapter 7 Solutions
Principles of Physics: A Calculus-Based Text
Ch. 7.1 - By what transfer mechanisms does energy enter and...Ch. 7.1 - Consider a block sliding over a horizontal surface...Ch. 7.2 - Prob. 7.3QQCh. 7.2 - Prob. 7.4QQCh. 7.4 - Prob. 7.5QQCh. 7 - You hold a slingshot at arms length, pull the...Ch. 7 - An athlete jumping vertically on a trampoline...Ch. 7 - Prob. 3OQCh. 7 - Two children stand on a platform at the top of a...Ch. 7 - Answer yes or no to each of the following...
Ch. 7 - A ball of clay falls freely to the hard floor. It...Ch. 7 - What average power is generated by a 70.0-kg...Ch. 7 - In a laboratory model of cars skidding to a stop,...Ch. 7 - At the bottom of an air track tilted at angle , a...Ch. 7 - One person drops a ball from the top of a building...Ch. 7 - Prob. 2CQCh. 7 - Does everything have energy? Give the reasoning...Ch. 7 - Prob. 4CQCh. 7 - Prob. 5CQCh. 7 - Prob. 6CQCh. 7 - A block is connected to a spring that is suspended...Ch. 7 - Consider the energy transfers and transformations...Ch. 7 - Prob. 9CQCh. 7 - Prob. 10CQCh. 7 - Prob. 1PCh. 7 - Prob. 2PCh. 7 - Review. A bead slides without friction around a...Ch. 7 - At 11:00 a.m, on September 7, 2001, more than one...Ch. 7 - A block of mass 0.250 kg is placed on top of a...Ch. 7 - A block of mass m = 5.00 kg is released from point...Ch. 7 - Two objects are connected by a light string...Ch. 7 - Prob. 8PCh. 7 - Prob. 9PCh. 7 - Prob. 10PCh. 7 - Prob. 11PCh. 7 - A crate of mass 10.0 kg is pulled up a rough...Ch. 7 - Prob. 13PCh. 7 - Prob. 14PCh. 7 - A block of mass m = 2.00 kg is attached to a...Ch. 7 - Prob. 16PCh. 7 - A smooth circular hoop with a radius of 0.500 m is...Ch. 7 - Prob. 18PCh. 7 - Prob. 19PCh. 7 - As shown in Figure P7.20, a green bead of mass 25...Ch. 7 - A 5.00-kg block is set into motion up an inclined...Ch. 7 - The coefficient of friction between the block of...Ch. 7 - Prob. 23PCh. 7 - Prob. 24PCh. 7 - Prob. 25PCh. 7 - Prob. 26PCh. 7 - A child of mass m starts from rest and slides...Ch. 7 - The electric motor of a model train accelerates...Ch. 7 - Prob. 29PCh. 7 - Prob. 30PCh. 7 - Prob. 31PCh. 7 - Sewage at a certain pumping station is raised...Ch. 7 - Prob. 33PCh. 7 - Prob. 34PCh. 7 - Prob. 35PCh. 7 - Prob. 36PCh. 7 - Prob. 37PCh. 7 - Prob. 38PCh. 7 - Prob. 39PCh. 7 - Prob. 40PCh. 7 - A loaded ore car has a mass of 950 kg and rolls on...Ch. 7 - Prob. 42PCh. 7 - A certain automobile engine delivers 2.24 104 W...Ch. 7 - Prob. 44PCh. 7 - A small block of mass m = 200 g is released from...Ch. 7 - Prob. 46PCh. 7 - Prob. 47PCh. 7 - Prob. 48PCh. 7 - Prob. 49PCh. 7 - Prob. 50PCh. 7 - Prob. 51PCh. 7 - Prob. 52PCh. 7 - Jonathan is riding a bicycle and encounters a hill...Ch. 7 - Prob. 54PCh. 7 - A horizontal spring attached to a wall has a force...Ch. 7 - Prob. 56PCh. 7 - Prob. 57PCh. 7 - Prob. 58PCh. 7 - Prob. 59PCh. 7 - Prob. 60PCh. 7 - Prob. 61PCh. 7 - Prob. 62PCh. 7 - Make an order-of-magnitude estimate of your power...Ch. 7 - Prob. 64PCh. 7 - Prob. 65PCh. 7 - Review. As a prank, someone has balanced a pumpkin...Ch. 7 - Review. The mass of a car is 1 500 kg. The shape...Ch. 7 - A 1.00-kg object slides to the right on a surface...Ch. 7 - A childs pogo stick (Fig. P7.69) stores energy in...Ch. 7 - Prob. 70PCh. 7 - Prob. 71PCh. 7 - Prob. 72PCh. 7 - A block of mass m1 = 20.0 kg is connected to a...Ch. 7 - Prob. 74PCh. 7 - Prob. 75PCh. 7 - Prob. 76PCh. 7 - Prob. 77PCh. 7 - Prob. 78PCh. 7 - A block of mass 0.500 kg is pushed against a...Ch. 7 - A pendulum, comprising a light string of length L...Ch. 7 - Jane, whose mass is 50.0 kg, needs to swing across...Ch. 7 - A roller-coaster car shown in Figure P7.82 is...Ch. 7 - Prob. 83P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- pls help on thesearrow_forward20. Two small conducting spheres are placed on top of insulating pads. The 3.7 × 10-10 C sphere is fixed whie the 3.0 × 107 C sphere, initially at rest, is free to move. The mass of each sphere is 0.09 kg. If the spheres are initially 0.10 m apart, how fast will the sphere be moving when they are 1.5 m apart?arrow_forwardpls help on allarrow_forward
- 19. Mount Everest, Earth's highest mountain above sea level, has a peak of 8849 m above sea level. Assume that sea level defines the height of Earth's surface. (re = 6.38 × 106 m, ME = 5.98 × 1024 kg, G = 6.67 × 10 -11 Nm²/kg²) a. Calculate the strength of Earth's gravitational field at a point at the peak of Mount Everest. b. What is the ratio of the strength of Earth's gravitational field at a point 644416m below the surface of the Earth to a point at the top of Mount Everest? C. A tourist watching the sunrise on top of Mount Everest observes a satellite orbiting Earth at an altitude 3580 km above his position. Determine the speed of the satellite.arrow_forwardpls help on allarrow_forwardpls help on allarrow_forward
- 6. As the distance between two charges decreases, the magnitude of the electric potential energy of the two-charge system: a) Always increases b) Always decreases c) Increases if the charges have the same sign, decreases if they have the opposite signs d) Increases if the charges have the opposite sign, decreases if they have the same sign 7. To analyze the motion of an elastic collision between two charged particles we use conservation of & a) Energy, Velocity b) Momentum, Force c) Mass, Momentum d) Energy, Momentum e) Kinetic Energy, Potential Energyarrow_forwardpls help on all asked questions kindlyarrow_forwardpls help on all asked questions kindlyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Kinetic Energy and Potential Energy; Author: Professor Dave explains;https://www.youtube.com/watch?v=g7u6pIfUVy4;License: Standard YouTube License, CC-BY