
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.SE, Problem 25EDRM
When isopropylidenecyclohexane is treated with strong acid at room temperature, isomerization occurs by the mechanism shown below to yield 1-isopropylcyclohexene:
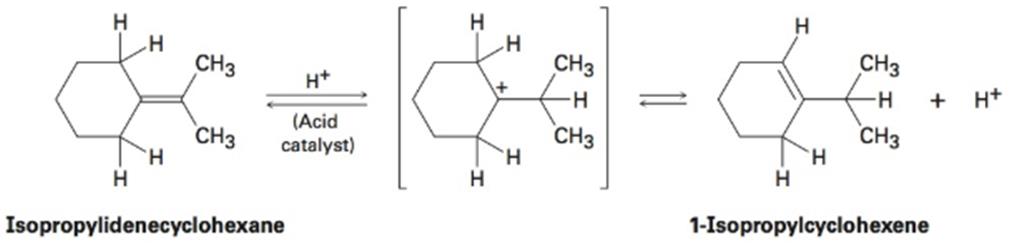
At equilibrium, the product mixture contains about 30% isopropyl-idenecyclohexane and about 70% 1-isopropylcyclohexene.
(a) What is an approximate value of Keq for the reaction?
(b) Since the reaction occurs slowly at room temperature, what is its approximate ∆G‡?
(c) Draw an energy diagram for the reaction.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
In the battery:Pt | H2 (g) | H+ (aq) | Fe2+ (aq) | FeIndicate the cathode and anode.
Write the equations that occur when the electrode Pb (s) | PbI2 (s) | KI (ac) in a galvanic cell. a) It functions as a positive electrode b) It functions as a negative electrode c) What is the ion with respect to which this electrode is reversible?
State the formula to find the electromotive force of a battery as a function of the potential of the anode and the cathode.
Chapter 6 Solutions
Organic Chemistry
Ch. 6.1 - Prob. 1PCh. 6.3 - Prob. 2PCh. 6.3 - Using curved fishhook arrows, propose a mechanism...Ch. 6.4 - Prob. 4PCh. 6.4 - An electrostatic potential map of boron...Ch. 6.5 - What product would you expect from reaction of...Ch. 6.5 - Reaction of HBr with 2-methylpropene yields...Ch. 6.6 - Prob. 8PCh. 6.6 - Predict the products of the following polar...Ch. 6.7 - Which reaction is more energetically favored, one...
Ch. 6.7 - Prob. 11PCh. 6.9 - Which reaction is faster, one with ∆G‡ = +45...Ch. 6.10 - Prob. 13PCh. 6.SE - Prob. 14VCCh. 6.SE - Prob. 15VCCh. 6.SE - Prob. 16VCCh. 6.SE - Look at the following energy diagram: (a) Is...Ch. 6.SE - Look at the following energy diagram for an...Ch. 6.SE - What is the difference between a transition state...Ch. 6.SE - Prob. 20EDRMCh. 6.SE - Prob. 21EDRMCh. 6.SE - Draw an energy diagram for a two-step exergonic...Ch. 6.SE - Draw an energy diagram for a reaction with keq =...Ch. 6.SE - The addition of water to ethylene to yield ethanol...Ch. 6.SE - When isopropylidenecyclohexane is treated with...Ch. 6.SE - Prob. 26EDRMCh. 6.SE - Draw the electron-pushing mechanism for each...Ch. 6.SE - Draw the complete mechanism for each polar...Ch. 6.SE - Prob. 29EDRMCh. 6.SE - Identify the functional groups in the following...Ch. 6.SE - Identify the following reactions as additions,...Ch. 6.SE - Identify the likely electrophilic and nucleophilic...Ch. 6.SE - For each reaction below identify the electrophile...Ch. 6.SE - Prob. 34APCh. 6.SE - Follow the flow of electrons indicated by the...Ch. 6.SE - Prob. 36APCh. 6.SE - Prob. 37APCh. 6.SE - Despite the limitations of radical chlorination of...Ch. 6.SE - Prob. 39APCh. 6.SE - Answer question 6-39 taking all stereoisomers into...Ch. 6.SE - Prob. 41APCh. 6.SE - Prob. 42APCh. 6.SE - Prob. 43APCh. 6.SE - The reaction of hydroxide ion with chloromethane...Ch. 6.SE - Prob. 45APCh. 6.SE - Ammonia reacts with acetyl chloride (CH3COCl) to...Ch. 6.SE - The naturally occurring molecule α-terpineol is...Ch. 6.SE - Prob. 48APCh. 6.SE - Prob. 49APCh. 6.SE - Draw the structures of the two carbocation...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why are normal electrode potentials also called relative electrode potentials?arrow_forwardEasily differentiate between electrochemical potential and Galvani potential.arrow_forwardConstruct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY