
An ore containing 90 wt% MgSO4(H2O and the balance insoluble minerals is fed to a dissolution tank at a rate of 60,000 lbm/h along with fresh water and a recycle stream. The tank contents are heated to 120°F, causing all of the magnesium sulfate monohydrate in the ore to dissolve, forming a solution 10°F above saturation. The resulting slurry of the insoluble minerals in MgSO4 solution is pumped to a heated ?lter, where a wet ?lter cake is separated from a solids-free ?ltrate. The ?lter cake retains 5 lbm of solution per 100 lbm of solids. The ?ltrate is sent to a crystallizer in which the temperature is reduced to 50°F, producing a slurry of MgSO4(7H2O crystals in a saturated solution that is sent to another ?lter. The product ?lter cake contains all of the crystals and entrained solution in a ratio of 5 lbm solution per 100 lbm crystals. The ?ltrate from this ?lter is returned to the dissolution tank as the recycle stream.
Solubility data: Saturated magnesium sulfate solutions at 110°F and 50°F contain 32 wt% MgSO4 and 23 wt% MgSO4, respectively.
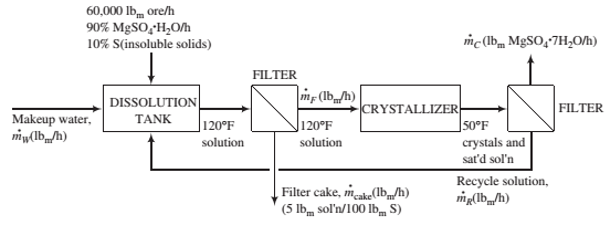
(a) Explain why the solution is ?rst heated (in the dissolution tank) and ?ltered and then cooled (in the crystallizer) and ?ltered.
(b) Calculate the production rate of crystals and the required feed rate of fresh water to the dissolution tank. (Note: Don‘t forget to include water of hydration when you write a mass balance on water.)
(c) Calculate the ratio lbm recycle/lbm makeup water.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Elementary Principles of Chemical Processes, Binder Ready Version
Additional Engineering Textbook Solutions
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Modern Database Management
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
Web Development and Design Foundations with HTML5 (8th Edition)
Mechanics of Materials (10th Edition)
Java: An Introduction to Problem Solving and Programming (8th Edition)
- Q2/ An adsorption study is set up in laboratory by adding a known amount of activated carbon to six which contain 200 mL of an industrial waste. An additional flask containing 200 mL of waste but no c is run as a blank. Plot the Langmuir isotherm and determine the values of the constants. Flask No. Mass of C (mg) Volume in Final COD Flask (mL) (mg C/L) 1 804 200 4.7 2 668 200 7.0 3 512 200 9.31 4 393 200 16.6 C 5 313 200 32.5 6 238 200 62.8 7 0 200 250arrow_forwardمشر on ۲/۱ Two rods (fins) having same dimensions, one made of brass(k=85 m K) and the other of copper (k = 375 W/m K), having one of their ends inserted into a furnace. At a section 10.5 cm a way from the furnace, the temperature brass rod 120°C. Find the distance at which the same temperature would be reached in the copper rod ? both ends are exposed to the same environment. 22.05 ofthearrow_forward4.59 Using the unilateral z-transform, solve the following difference equations with the given initial conditions. (a) y[n]-3y[n-1] = x[n], with x[n] = 4u[n], y[− 1] = 1 (b) y[n]-5y[n-1]+6y[n-2]= x[n], with x[n] = u[n], y[-1] = 3, y[-2]= 2 Ans. (a) y[n] = -2+9(3)", n ≥ -1 (b) y[n]=+8(2)" - (3)", n ≥ -2arrow_forward
- (30) 6. In a process design, the following process streams must be cooled or heated: Stream No mCp Temperature In Temperature Out °C °C kW/°C 1 5 350 270 2 9 270 120 3 3 100 320 4 5 120 288 Use the MUMNE algorithm for heat exchanger networks with a minimum approach temperature of 20°C. (5) a. Determine the temperature interval diagram. (3) (2) (10) (10) b. Determine the cascade diagram, the pinch temperatures, and the minimum hot and cold utilities. c. Determine the minimum number of heat exchangers above and below the pinch. d. Determine a valid heat exchange network above the pinch. e. Determine a valid heat exchange network below the pinch.arrow_forwardUse this equation to solve it.arrow_forwardQ1: Consider the following transfer function G(s) 5e-s 15s +1 1. What is the study state gain 2. What is the time constant 3. What is the value of the output at the end if the input is a unit step 4. What is the output value if the input is an impulse function with amplitude equals to 3, at t=7 5. When the output will be 3.5 if the input is a unit steparrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





