
A liquid stream consisting of 12.5 mole% n-butane and the balance a heavy nonvolatile hydrocarbon is fed to the top of a stripping column, where it is contacted with an upward-?owing stream of nitrogen. The residual liquid leaves the bottom of the column containing all of the heavy hydrocarbon, 5% of the butane entering the column, and a negligible amount of dissolved nitrogen.
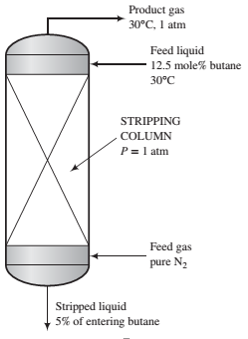
(a) The highest possible butane mole fraction in the exiting gas is that in equilibrium with the butane in the entering liquid (a condition that would require an in?nitely tall column to achieve). Using Raoult‘s law to relate the mole fractions of butane in the entering liquid and exiting gas, calculate the molar feed-stream ratio (mol gas fed/mol liquid fed) corresponding to this limiting condition.
(b) Suppose the actual mole fraction of butane in the exit gas is 80% of its theoretical maximum value and the percentage stripped (95%) is the same as in Part (a). Calculate the ratio (mol gas fed/mol liquid fed) for this case.
(c) Increasing the nitrogen feed rate for a given liquid feed rate and butane recovery decreases the cost of the process in one way and increases it in another. Explain. What would you have to know to determine the most cost-effective value of the gas/liquid feed ratio?
Trending nowThis is a popular solution!

Chapter 6 Solutions
Elementary Principles of Chemical Processes, Binder Ready Version
Additional Engineering Textbook Solutions
Web Development and Design Foundations with HTML5 (8th Edition)
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
BASIC BIOMECHANICS
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
- please, provide me the solution with detailsarrow_forwardPlease, provide me the solution with details and plot.arrow_forwardQ2/ An adsorption study is set up in laboratory by adding a known amount of activated carbon to six which contain 200 mL of an industrial waste. An additional flask containing 200 mL of waste but no c is run as a blank. Plot the Langmuir isotherm and determine the values of the constants. Flask No. Mass of C (mg) Volume in Final COD Flask (mL) (mg C/L) 1 804 200 4.7 2 668 200 7.0 3 512 200 9.31 4 393 200 16.6 C 5 313 200 32.5 6 238 200 62.8 7 0 200 250arrow_forward
- مشر on ۲/۱ Two rods (fins) having same dimensions, one made of brass(k=85 m K) and the other of copper (k = 375 W/m K), having one of their ends inserted into a furnace. At a section 10.5 cm a way from the furnace, the temperature brass rod 120°C. Find the distance at which the same temperature would be reached in the copper rod ? both ends are exposed to the same environment. 22.05 ofthearrow_forward4.59 Using the unilateral z-transform, solve the following difference equations with the given initial conditions. (a) y[n]-3y[n-1] = x[n], with x[n] = 4u[n], y[− 1] = 1 (b) y[n]-5y[n-1]+6y[n-2]= x[n], with x[n] = u[n], y[-1] = 3, y[-2]= 2 Ans. (a) y[n] = -2+9(3)", n ≥ -1 (b) y[n]=+8(2)" - (3)", n ≥ -2arrow_forward(30) 6. In a process design, the following process streams must be cooled or heated: Stream No mCp Temperature In Temperature Out °C °C kW/°C 1 5 350 270 2 9 270 120 3 3 100 320 4 5 120 288 Use the MUMNE algorithm for heat exchanger networks with a minimum approach temperature of 20°C. (5) a. Determine the temperature interval diagram. (3) (2) (10) (10) b. Determine the cascade diagram, the pinch temperatures, and the minimum hot and cold utilities. c. Determine the minimum number of heat exchangers above and below the pinch. d. Determine a valid heat exchange network above the pinch. e. Determine a valid heat exchange network below the pinch.arrow_forward
- Use this equation to solve it.arrow_forwardQ1: Consider the following transfer function G(s) 5e-s 15s +1 1. What is the study state gain 2. What is the time constant 3. What is the value of the output at the end if the input is a unit step 4. What is the output value if the input is an impulse function with amplitude equals to 3, at t=7 5. When the output will be 3.5 if the input is a unit steparrow_forwardgive me solution math not explinarrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





