
Elementary Principles of Chemical Processes, Binder Ready Version
4th Edition
ISBN: 9781118431221
Author: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6, Problem 6.79P
A two-unit process is used to separate H2S from a gas containing 96% H2 and 4% H2S by volume. The H2S is absorbed in a solvent, which is then regenerated by air in a stripping column. The Henry’s law constant for the absorption of H2S in the solvent at 0°C is 22 atm/mole fraction.
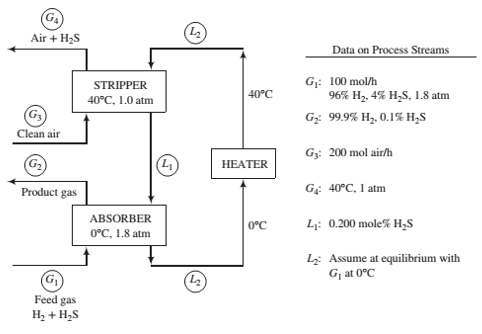
- Brie?y explain in your own words the functions of the three process units. Include in your explanation the purpose of the air in the stripper and the reason the stripper operates at a higher temperature than the absorber.
- Calculate the molar ?ow rate of pure solvent and the volumetric ?ow rate of the gas at G4, neglecting evaporation of solvent in both columns. (See ?owchart.) Exploratory Exercise—Research and Discover (c) The objective of the process described above is to produce puri?ed hydrogen. However, in doing so the process also generates an effluent stream containing H 2S. Identify at least three concerns with simply releasing this stream into the air. Suggest an add-on to the process that allays as many of these concerns as possible. Calculate the mass per 100 mol of G 1 of any reactant required by the add-on. Identify any new concerns created by the add-on.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
14.9. A forward feed double-effect vertical
evaporator, with equal heating areas in
each effect, is fed with 5 kg/s of a liquor
of specific heat capacity of 4.18 kJ/kg K.
and with no boiling point rise, so that 50
per cent of the feed liquor is evaporated.
The overall heat transfer coefficient in the
second effect is 75 per cent of that in the
first effect. Steam is fed at 395 K and the
boiling point in the second effect is 373 K.
The feed is heated by an external heater to
the boiling point in the first effect.
It is decided to bleed off 0.25 kg/s of
vapour from the vapour line to the second
effect for use in another process. If the
feed is still heated to the boiling point of
the first effect by external means, what
will be the change in steam consumption
of the evaporator unit? For the purpose of
calculation, the latent heat of the vapours
and of the steam may both be taken as
2230 kJ/kg
Example(3):
It is desired to design a double effect
evaporator for concentrating a certain
caustic soda solution from 12.5wt% to
40wt%. The feed at 50°C enters the first
evaporator at a rate of 2500kg/h. Steam
at atmospheric pressure is being used
for the said purpose. The second effect
is operated under 600mmHg vacuum. If
the overall heat transfer coefficients of
the two stages are 1952 and 1220kcal/
m2.h.°C. respectively, determine the heat
transfer area of each effect. The BPR will
be considered and present for the both
effect
5:49
العنوان
ose only
Q
Example (7):
Determine the heating surface area
개
required for the production of 2.5kg/s of
50wt% NaOH solution from 15 wt% NaOH
feed solution which entering at 100 oC to
a single effect evaporator. The steam is
available as saturated at 451.5K and the
boiling point rise (boiling point evaluation)
of 50wt% solution is 35K. the overall
heat transfer coefficient is 2000 w/m²K.
The pressure in the vapor space of the
evaporator at atmospheric pressure. The
solution has a specific heat of 4.18kJ/
kg.K. The enthalpy of vaporization under
these condition is 2257kJ/kg
Example (6):
5:48
An evaporator is concentrating F kg/h at
311K of a 20wt% solution of NaOH to 50wt
%. The saturated steam used for heating is
at 399.3K. The pressure in the vapor space
of the evaporator is 13.3 KPa abs. The
5:48
1
J
۲/۱
ostr
Chapter 6 Solutions
Elementary Principles of Chemical Processes, Binder Ready Version
Ch. 6 - Ten mL of pure liquid water in a cylinder with a...Ch. 6 - A quantity of methyl acetate is placed in an open,...Ch. 6 - Ethyl alcohol has a vapor pressure of 20.0 mm Hg...Ch. 6 - Prob. 6.4PCh. 6 - Prob. 6.5PCh. 6 - Prob. 6.6PCh. 6 - Prob. 6.7PCh. 6 - Prob. 6.8PCh. 6 - Prob. 6.9PCh. 6 - Prob. 6.10P
Ch. 6 - Prob. 6.11PCh. 6 - Prob. 6.12PCh. 6 - Prob. 6.13PCh. 6 - Air at 50% relative humidity is cooled...Ch. 6 - Prob. 6.15PCh. 6 - Prob. 6.16PCh. 6 - Air at 90°C and 1.00 atm (absolute) contains 10.0...Ch. 6 - When fermentation units are operated with high...Ch. 6 - When you step out of a shower, the temperature in...Ch. 6 - A fuel cell is an electrochemical device in which...Ch. 6 - Prob. 6.21PCh. 6 - Prob. 6.22PCh. 6 - Prob. 6.23PCh. 6 - Prob. 6.24PCh. 6 - Prob. 6.25PCh. 6 - Prob. 6.26PCh. 6 - Prob. 6.27PCh. 6 - Prob. 6.28PCh. 6 - An air conditioner is designed to bring 10.000...Ch. 6 - Prob. 6.30PCh. 6 - Prob. 6.31PCh. 6 - Prob. 6.32PCh. 6 - A gas stream containing 40.0 mole% hydrogen, 35.0%...Ch. 6 - Prob. 6.34PCh. 6 - In the manufacture of an active pharmaceutical...Ch. 6 - Prob. 6.36PCh. 6 - In the ?nal stage of the manufacturing process...Ch. 6 - Prob. 6.38PCh. 6 - A fuel gas containing methane and ethane is burned...Ch. 6 - A mixture of propane and butane is burned with...Ch. 6 - An important parameter in the design of gas...Ch. 6 - A liquid stream consisting of 12.5 mole% n-butane...Ch. 6 - Nitric acid is a chemical intermediate primarily...Ch. 6 - Prob. 6.44PCh. 6 - Sulfur trioxide (SO3) dissolves in and reacts with...Ch. 6 - State whether you would use Raoult’s law or Henrys...Ch. 6 - A gas containing nitrogen, benzene, and toluene is...Ch. 6 - Prob. 6.48PCh. 6 - Prob. 6.49PCh. 6 - A conelation for methane solubility in...Ch. 6 - Prob. 6.51PCh. 6 - The constituent partial pressures of a gas in...Ch. 6 - Prob. 6.53PCh. 6 - Prob. 6.54PCh. 6 - Prob. 6.55PCh. 6 - Prob. 6.56PCh. 6 - Prob. 6.57PCh. 6 - Prob. 6.58PCh. 6 - Nitrogen is bubbled through a liquid mixture that...Ch. 6 - Prob. 6.60PCh. 6 - Prob. 6.61PCh. 6 - Prob. 6.62PCh. 6 - The feed to a distillation column (sketched below)...Ch. 6 - Prob. 6.64PCh. 6 - Prob. 6.65PCh. 6 - Prob. 6.66PCh. 6 - Prob. 6.67PCh. 6 - Prob. 6.68PCh. 6 - Prob. 6.69PCh. 6 - Prob. 6.70PCh. 6 - A methanol-water feed stream is introduced to a...Ch. 6 - Prob. 6.72PCh. 6 - In this problem you will use a spreadsheet to...Ch. 6 - Prob. 6.74PCh. 6 - Prob. 6.75PCh. 6 - Prob. 6.76PCh. 6 - Acetaldehyde is synthesized by the catalytic...Ch. 6 - Dehydration of natural gas is necessary to prevent...Ch. 6 - A two-unit process is used to separate H2S from a...Ch. 6 - Prob. 6.80PCh. 6 - Prob. 6.81PCh. 6 - Prob. 6.82PCh. 6 - Prob. 6.83PCh. 6 - A solution containing 100 lbm KNO3/100 Ibm H2O at...Ch. 6 - A 10.0 wt% aqueous solution of sodium chloride is...Ch. 6 - Potassium dichromate (K2Cr2O7) is to be recovered...Ch. 6 - Prob. 6.87PCh. 6 - Prob. 6.88PCh. 6 - Sodium bicarbonate is synthesized by reacting...Ch. 6 - An ore containing 90 wt% MgSO4(H2O and the balance...Ch. 6 - An aqueous waste stream leaving a process contains...Ch. 6 - A solution of diphenyl (MW = 154.2) in benzene is...Ch. 6 - An aqueous solution of urea (MW = 60.06) freezes...Ch. 6 - Prob. 6.94PCh. 6 - Derive Equation 6.54 for the boiling-point...Ch. 6 - Prob. 6.96PCh. 6 - A stream of 5.00 wt% oleic acid in cottonseed oil...Ch. 6 - Benzene and hexane are being considered as...Ch. 6 - Acetone is lo be extracted with n-hexane from a...Ch. 6 - Prob. 6.100PCh. 6 - Prob. 6.101PCh. 6 - Five kilograms of a 30 wt% acetone70% water...Ch. 6 - An aqueous acetone solution is fed at a rate of...Ch. 6 - Prob. 6.104PCh. 6 - Prob. 6.105PCh. 6 - Air at 25°C and 1 atm with a relative humidity of...Ch. 6 - Prob. 6.107PCh. 6 - Prob. 6.108PCh. 6 - Various amounts of activated carbon were added to...
Additional Engineering Textbook Solutions
Find more solutions based on key concepts
The class SalariedEmployee inherits both of the functions getName and printCheck (among other things) from the ...
Problem Solving with C++ (10th Edition)
Referring back to Questions 3 of Section 2.3, if the machine used the pipeline technique discussed in the text,...
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
What is the value of x after each of the following statements is executed? double x = Math.abs(0.0);
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Distinguish among data definition commands, data manipulation commands, and data control commands.
Modern Database Management
This is a single piece of data within a record. a. field b. variable c. delimiter d. subrecord
Starting Out with Python (4th Edition)
What are some common objectives of surfacing operations?
Degarmo's Materials And Processes In Manufacturing
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Example 8: 900 Kg dry solid per hour is dried in a counter current continues dryer from 0.4 to 0.04 Kg H20/Kg wet solid moisture content. The wet solid enters the dryer at 25 °C and leaves at 55 °C. Fresh air at 25 °C and 0.01Kg vapor/Kg dry air is mixed with a part of the moist air leaving the dryer and heated to a temperature of 130 °C in a finned air heater and enters the dryer with 0.025 Kg/Kg alry air. Air leaving the dryer at 85 °C and have a humidity 0.055 Kg vaper/Kg dry air. At equilibrium the wet solid weight is 908 Kg solid per hour. *=0.0088 Calculate:- Heat loss from the dryer and the rate of fresh air. Take the specific heat of the solid and moisture are 980 and 4.18J/Kg.K respectively, A. =2500 KJ/Kg. Humid heat at 0.01 Kg vap/Kg dry=1.0238 KJ/Kg. "C. Humid heat at 0.055 Kg/Kg 1.1084 KJ/Kg. "C 5:42 Oarrow_forwardQ1: From the Figure below for (=0.2 find the following 1. Rise Time 2. Time of oscillation 3. Overshoot value 4. Maximum value 5. When 1.2 which case will be? 1.6 1.4 1.2 12 1.0 |=0.8- 0.6 0.4 0.8 0.2- 0.6 0.4 0.2 1.2 = 1.0 0 2 4 6 8 10 10 t/Tarrow_forwardPlease, I need solution in detailsarrow_forward
- please, I need solution in detailsarrow_forwardplease, I need solution in detailsarrow_forwardA system, in a closed container, consists of an unknown number of components and three phases. You are told that the system is fully defined by giving you only one mole fraction! What is the number components that is present? 3 1 2 The question is ill-posed.arrow_forward
- A mixture of 2 components in 2 phases are present. You are given the temperature and mole fraction. How many additional variables can be specified before the system is completely determined? none 2 the system is overspecified 1 3arrow_forwardAt a Pressure of 600 mm Hg, match the substance with the boiling temperature. 54.69°C 1. n-Pentane 49.34°C 2. n-Hexane 3. Acetone 29.32°C く 61.40°C 4. Chloroformarrow_forwardA mixture of oil and gas flows through a horizontal pipe with an inside diameter of 150 mm. The respective volumetric flow rates for the oil and gas are 0.015 and 0.29 m³s-1. Determine the gas void frac- tion and the average velocities of the oil and gas. The friction factor may be assumed to be 0.0045. The gas has a density of 2.4 kgm³ and viscosity of 1 x 10-5 Nsm-2. The oil has a density of 810 kgm³ and density of 0.82 Nsm². Answer: 0.79, 20.8 ms-1, 4 ms-1arrow_forward
- 4. An experimental test rig is used to examine two-phase flow regimes in horizontal pipelines. A particular experiment involved uses air and water at a temperature of 25°C, which flow through a horizontal glass tube with an internal diameter of 25.4 mm and a length of 40 m. Water is admitted at a controlled rate of 0.026 kgs at one end and air at a rate of 5 x 104 kgs in the same direction. The density of water is 1000 kgm³, and the density of air is 1.2 kgm3. Determine the mass flow rate, the mean density, gas void fraction, and the superficial velocities of the air and water. Answer: 0.02605 kgs 1, 61.1 kgm³, 0.94, 0.822 ms-1, 0.051 ms-1arrow_forward1. Determine the range of mean density of a mixture of air in a 50:50 oil-water liquid phase across a range of gas void fractions. The den- sity of oil is 900 kgm³, water is 1000 kgm³, and gas is 10 kgm³. 2. Describe, with the use of sketches, the various flow regimes that can exist in a vertical pipe carrying two-phase flow (liquid and gas).arrow_forwardA mixture of high pressure water and steam at a rate of 0.5 kgs-¹ flows up a vertical tube with an inside diameter of 25.4 mm at a pres- sure 22 bar. Determine the type of flow if the mass quality is 1%. The density of the water is 845 kgm³, the density of steam is 10.8 kgm³, and the viscosity of the water is 1.24 x 104 Nsm2. Answer: Slug flowarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The
Mod-01 Lec-23 Degrees of freedom analysis; Author: nptelhrd;https://www.youtube.com/watch?v=c4h85JjrkzQ;License: Standard YouTube License, CC-BY
Introduction to Degrees of Freedom; Author: LearnChemE;https://www.youtube.com/watch?v=tW1ft4y5fQY;License: Standard Youtube License