
An important parameter in the design of gas absorbers is the ratio of the ?ow rate of the feed liquid to that of the feed gas. The lower the value of this ratio, the lower the cost of the solvent required to process a given quantity of gas but the taller the absorber must be to achieve a speci?ed separation.
Propane is recovered from a 7 mole% propane—93% nitrogen mixture by contacting the mixture with liquid n-decane. An insigni?cant amount of decane is vaporized in the process, and 98.5% of the propane entering the unit is absorbed.
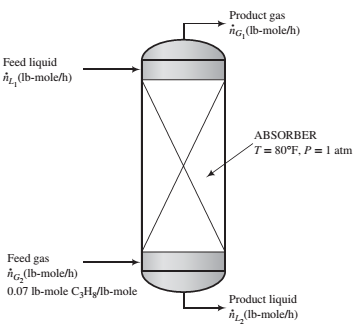
(a) The highest possible propane mole fraction in the exiting liquid is that in equilibrium with the propane mole fraction in the feed gas (a condition requiring an in?nitely tall column). Using Raoult’s law to relate the mole fractions of propane in the feed gas and liquid, calculate the ratio 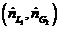 corresponding to this limiting condition.
corresponding to this limiting condition.
(b) Suppose the actual feed ratio 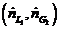 is 1.2 times the value calculated in Part (a) and the percentage of the entering propane absorbed is the same (98.5%). Calculate the mole fraction of propane in the exiting liquid.
is 1.2 times the value calculated in Part (a) and the percentage of the entering propane absorbed is the same (98.5%). Calculate the mole fraction of propane in the exiting liquid.
(c) What are the costs and bene?ts associated with increasing 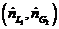 from its minimum value [the value calculated in Part (a)]? What would you have to know to determine the most cost−effective value of this ratio?
from its minimum value [the value calculated in Part (a)]? What would you have to know to determine the most cost−effective value of this ratio?
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Elementary Principles of Chemical Processes, Binder Ready Version
Additional Engineering Textbook Solutions
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
Starting Out with Python (4th Edition)
Introduction To Programming Using Visual Basic (11th Edition)
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
Modern Database Management
- Q1.B. Make a comparison between current control PWM rectifier in the abc reference frame and dq reference frame.arrow_forwardstep by steparrow_forwardThe power out of an adiabatic steam turbine is 5 MW and the steam enters turbine at 2 MPa and velocity of 50 m/s, specific enthalpy (h) of 3248 kJ/kg. The elevation of the inlet is 10 m higher than at the datum. The vapor mixture exits at 15 kPa and a velocity of 180 m/s, specific enthalpy (h) of 2361.01 kJ/kg. The elevation of the exit is 6 m higher than at the datum. Let g = 9.81 m/s². Assuming the ideal gas model and R = 0.462 KJ/(kg.K). The steam specific heat ratio is 1.283. Calculate:arrow_forward
- The power out of an adiabatic steam turbine is 5 MW and the steam enters turbine at 2 MPa and velocity of 50 m/s, specific enthalpy (h) of 3248 kJ/kg. The elevation of the inlet is 10 m higher than at the datum. The vapor mixture exits at 15 kPa and a velocity of 180 m/s, specific enthalpy (h) of 2361.01 kJ/kg. The elevation of the exit is 6 m higher than at the datum. Let g = 9.81 m/s². Assuming the ideal gas model and R = 0.462 KJ/(kg.K). The steam specific heat ratio is 1.283. Calculate:arrow_forwardThe power out of an adiabatic steam turbine is 5 MW and the steam enters turbine at 2 MPa and velocity of 50 m/s, specific enthalpy (h) of 3248 kJ/kg. The elevation of the inlet is 10 m higher than at the datum. The vapor mixture exits at 15 kPa and a velocity of 180 m/s, specific enthalpy (h) of 2361.01 kJ/kg. The elevation of the exit is 6 m higher than at the datum. Let g = 9.81 m/s². Assuming the ideal gas model and R = 0.462 KJ/(kg.K). The steam specific heat ratio is 1.283. Calculate:arrow_forwardO Consider a 0.8 m high and 0.5 m wide window with thickness of 8 mm and thermal conductivity of k = 0.78 W/m °C. For dry day, the temperature of outdoor is -10 °C and the inner room temperature is 20°C. Take the heat transfer coefficient on the inner and outer surface of the window to be h₁ = 10 W/m² °C and h₂ = 40 W/m² °C which includes the effects of insulation. Determine:arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





