
Concept explainers
(a)
Interpretation:
IUPAC name for the parent amine from which the given amine salt is formed has to be given.
Concept Introduction:
IUPAC rules for naming ammonium ions:
There are two rules that has to be followed while naming a positive ion (ammonium ion) and they are,
- In order to name an alkylamine, the ending of the name amine is changed from amine to ammonium ion.
- In order to name an
aromatic amine, the ending of the name “-e” is replaced by “-ium ion”.
- Longest carbon chain has to be identified that is attached to nitrogen atom.
- Suffix “-e” in name of the parent chain
alkane is replaced by “-amine”. - Numbering of the carbon chain is done from the end that is near the nitrogen atom.
- Point of attachment of the nitrogen atom in the carbon chain is indicated by a number before the parent chain name.
- In case if substituents are present, then the identity and location of substituents are appended to the front in the parent chain name.
If the compound contains two amine groups, then the suffix “-e” is replaced by diamine. Tertiary and secondary
Common name for amine is given in a single word. Primary amine is named as alkylamine. Secondary amine is named as alkylalkylamine. Tertiary amine is named as alkylalkylalkylamine.
(a)
Answer to Problem 6.58EP
Name of the parent amine is N-methylethanamine.
Explanation of Solution
Given amine salt is,
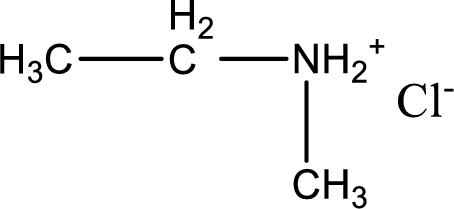
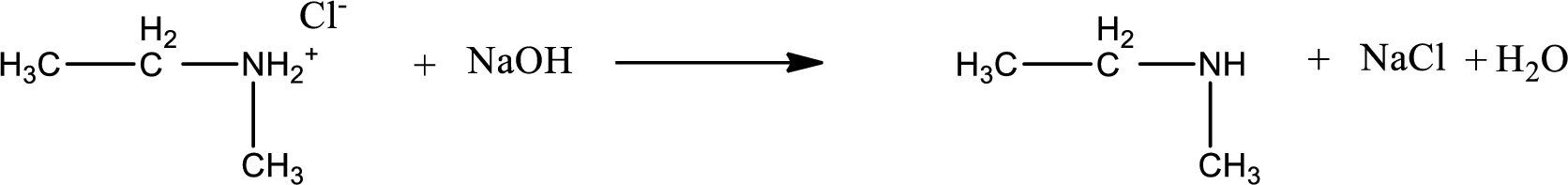
The parent amine can be found by deprotonating the amine salt. This can be accomplished by treating it with a strong base. The complete reaction can be given as,
Structure of the amine is,
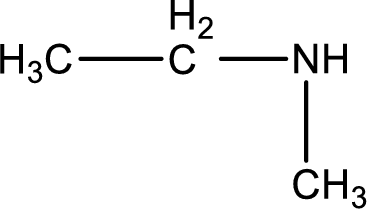
The longest carbon chain attached to the nitrogen atom is found to be containing two carbon atoms. Hence, the parent alkane is ethane. Amine is named by replacing the suffix “-e” in the parent alkane name with “-amine”. This gives the name as ethanamine.
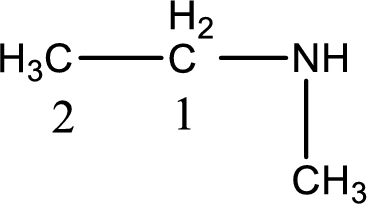
Numbering has to be given from the end that is near to the nitrogen atom. In this case, the nitrogen atom is attached to the carbon atom that is numbered 1. In this case numbering does not make sense as only two carbon atoms are present. Looking for substituent a methyl group is present on the nitrogen atom. This gives the IUPAC name of N-methylethanamine.
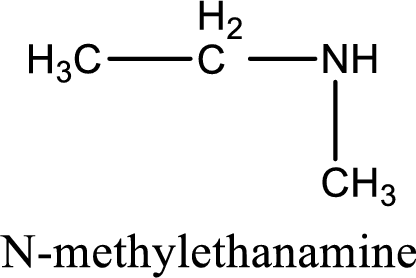
IUPAC name for the parent amine is given.
(b)
Interpretation:
IUPAC name for the parent amine from which the given amine salt is formed has to be given.
Concept Introduction:
IUPAC rules for naming ammonium ions:
There are two rules that has to be followed while naming a positive ion (ammonium ion) and they are,
- In order to name an alkylamine, the ending of the name amine is changed from amine to ammonium ion.
- In order to name an aromatic amine, the ending of the name “-e” is replaced by “-ium ion”.
IUPAC nomenclature for amine: There are about five rules to be followed in giving IUPAC name for an amine.
- Longest carbon chain has to be identified that is attached to nitrogen atom.
- Suffix “-e” in name of the parent chain alkane is replaced by “-amine”.
- Numbering of the carbon chain is done from the end that is near the nitrogen atom.
- Point of attachment of the nitrogen atom in the carbon chain is indicated by a number before the parent chain name.
- In case if substituents are present, then the identity and location of substituents are appended to the front in the parent chain name.
If the compound contains two amine groups, then the suffix “-e” is replaced by diamine. Tertiary and secondary amines are named as N-substituted primary amines.
Common name for amine is given in a single word. Primary amine is named as alkylamine. Secondary amine is named as alkylalkylamine. Tertiary amine is named as alkylalkylalkylamine.
(b)
Answer to Problem 6.58EP
Name of the parent amine is 1-butanamine.
Explanation of Solution
Given amine salt is
The parent amine can be found by deprotonating the amine salt. This can be accomplished by treating it with a strong base. The complete reaction can be given as,

Structure of the amine is,
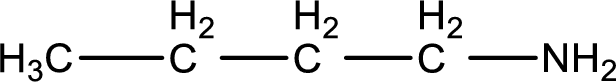
The longest carbon chain attached to the nitrogen atom is found to be containing four carbon atoms. Hence, the parent alkane is butane. Amine is named by replacing the suffix “-e” in the parent alkane name with “-amine”. This gives the name as butanamine.
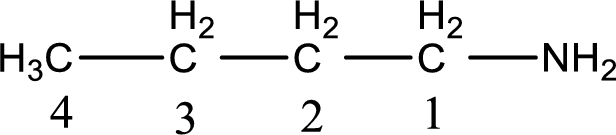
Numbering has to be given from the end that is near to the nitrogen atom. In this case, the nitrogen atom is attached to the carbon atom that is numbered 1. This has to be added to the name in front. This gives the IUPAC name of 1-butanamine.
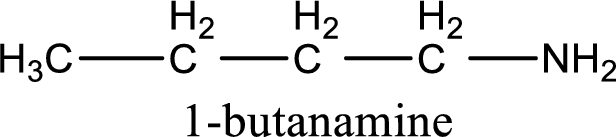
IUPAC name for the parent amine is given.
(c)
Interpretation:
IUPAC name for the parent amine from which the given amine salt is formed has to be given.
Concept Introduction:
IUPAC rules for naming ammonium ions:
There are two rules that has to be followed while naming a positive ion (ammonium ion) and they are,
- In order to name an alkylamine, the ending of the name amine is changed from amine to ammonium ion.
- In order to name an aromatic amine, the ending of the name “-e” is replaced by “-ium ion”.
IUPAC nomenclature for amine: There are about five rules to be followed in giving IUPAC name for an amine.
- Longest carbon chain has to be identified that is attached to nitrogen atom.
- Suffix “-e” in name of the parent chain alkane is replaced by “-amine”.
- Numbering of the carbon chain is done from the end that is near the nitrogen atom.
- Point of attachment of the nitrogen atom in the carbon chain is indicated by a number before the parent chain name.
- In case if substituents are present, then the identity and location of substituents are appended to the front in the parent chain name.
If the compound contains two amine groups, then the suffix “-e” is replaced by diamine. Tertiary and secondary amines are named as N-substituted primary amines.
Common name for amine is given in a single word. Primary amine is named as alkylamine. Secondary amine is named as alkylalkylamine. Tertiary amine is named as alkylalkylalkylamine.
(c)
Answer to Problem 6.58EP
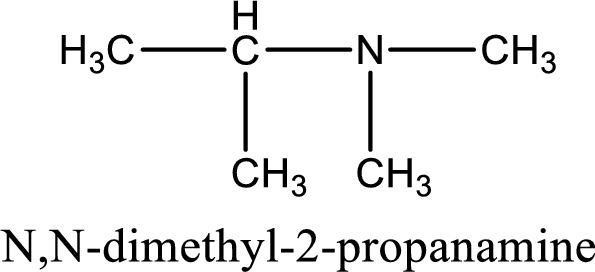
Name of the parent amine is N,N-dimethyl-2-propanamine.
Explanation of Solution
Given amine salt is,
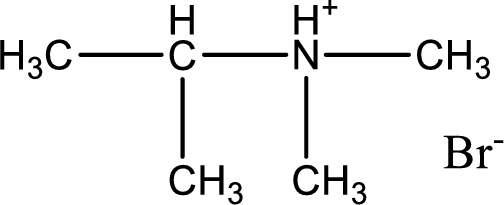
The parent amine can be found by deprotonating the amine salt. This can be accomplished by treating it with a strong base. The complete reaction can be given as,
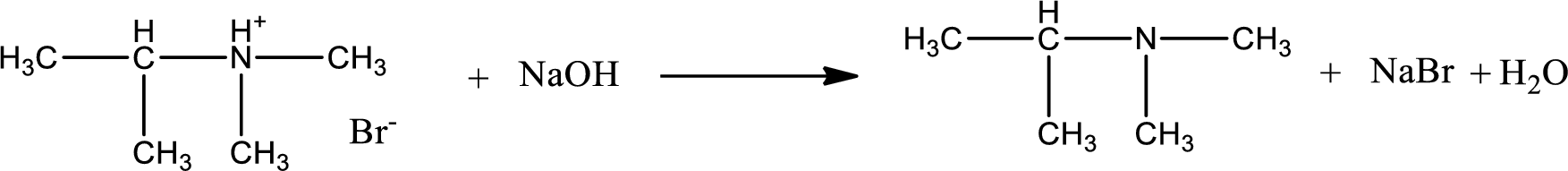
Structure of the amine is,
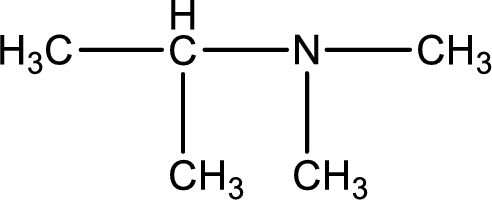
The longest carbon chain attached to the nitrogen atom is found to be containing three carbon atoms. Hence, the parent alkane is propane. Amine is named by replacing the suffix “-e” in the parent alkane name with “-amine”. This gives the name as propanamine.
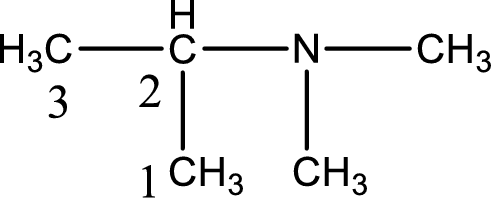
Numbering has to be given from the end that is near to the nitrogen atom. In this case, the nitrogen atom is attached to the carbon atom that is numbered 2. Looking for substituents, two methyl groups are present on the nitrogen atom. This gives the IUPAC name of N,N-dimethyl-2-propanamine.
IUPAC name for the parent amine is given.
(d)
Interpretation:
IUPAC name for the parent amine from which the given amine salt is formed has to be given.
Concept Introduction:
IUPAC rules for naming ammonium ions:
There are two rules that has to be followed while naming a positive ion (ammonium ion) and they are,
- In order to name an alkylamine, the ending of the name amine is changed from amine to ammonium ion.
- In order to name an aromatic amine, the ending of the name “-e” is replaced by “-ium ion”.
IUPAC nomenclature for amine: There are about five rules to be followed in giving IUPAC name for an amine.
- Longest carbon chain has to be identified that is attached to nitrogen atom.
- Suffix “-e” in name of the parent chain alkane is replaced by “-amine”.
- Numbering of the carbon chain is done from the end that is near the nitrogen atom.
- Point of attachment of the nitrogen atom in the carbon chain is indicated by a number before the parent chain name.
- In case if substituents are present, then the identity and location of substituents are appended to the front in the parent chain name.
If the compound contains two amine groups, then the suffix “-e” is replaced by diamine. Tertiary and secondary amines are named as N-substituted primary amines.
Common name for amine is given in a single word. Primary amine is named as alkylamine. Secondary amine is named as alkylalkylamine. Tertiary amine is named as alkylalkylalkylamine.
(d)
Answer to Problem 6.58EP
Name of the parent amine is N-methylphenylamine.
Explanation of Solution
Given amine salt is,
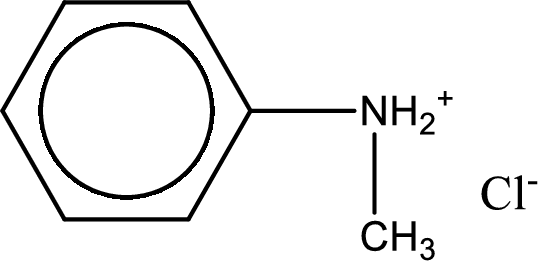
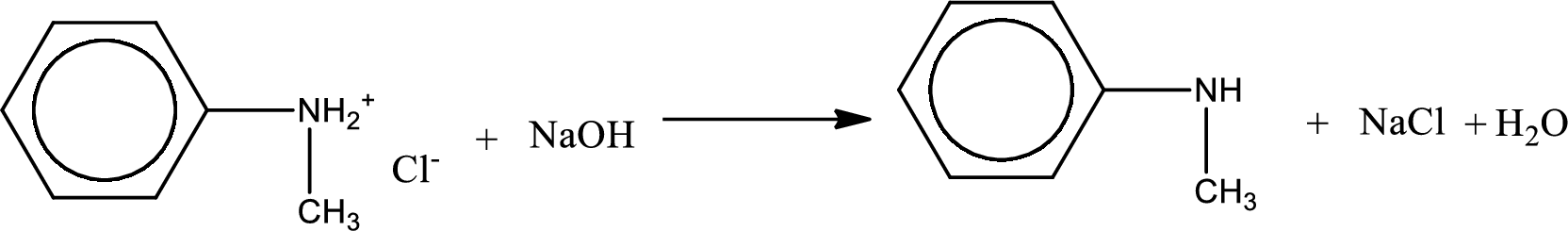
The parent amine can be found by deprotonating the amine salt. This can be accomplished by treating it with a strong base. The complete reaction can be given as,
Structure of the amine is,
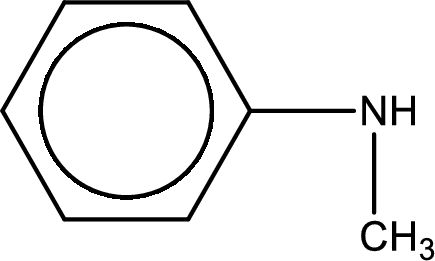
The longest carbon chain attached to the nitrogen atom is found to be containing six carbon cyclic chain. Hence, the parent is benzene. This has to be named as phenylamine.
Looking for substituents, one methyl group is present on the nitrogen atom. This gives the IUPAC name of N-methylphenylamine.
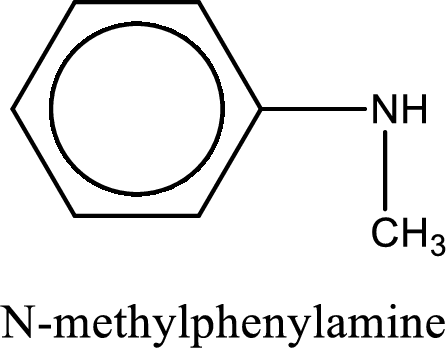
IUPAC name for the parent amine is given.
Want to see more full solutions like this?
Chapter 6 Solutions
Organic And Biological Chemistry
- In the decomposition reaction in solution B → C, only species C absorbs UV radiation, but neither B nor the solvent absorbs. If we call At the absorbance measured at any time, A0 the absorbance at the beginning of the reaction, and A∞ the absorbance at the end of the reaction, which of the expressions is valid? We assume that Beer's law is fulfilled.arrow_forward> You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products: 1. ☑ CI 2. H3O+ O Draw the missing reagent X you think will make this synthesis work in the drawing area below. If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. Explanation Check ? DO 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardDon't use ai to answer I will report you answerarrow_forward
- Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence pointarrow_forwardWhat is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward
- 7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forwardIndicate the compound formula: dimethyl iodide (propyl) sulfonium.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





