
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 6.24P
Draw the products of homolysis or heterolysis of each indicated bond. Use electronegativity
differences to decide on the location of charges in the heterolysis reaction. Classify each
carbon reactive intermediate as a radical, carbocation, or carbanion.
a.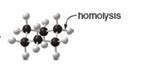 b.
b. 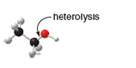
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please help me figure out the mechanism with arrows of the following reaction
Organic Functional Groups
Predicting the reactants or products of acetal hydrolysis
termine the structures of the missing organic molecules in the following reaction:
H*
H*
+ H₂O
Y
☑
Note: Molecules that share the same letter have the exact same structure.
In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw
that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure
Explanation
Check
@2
W
Click and drag to start drawing a structure.
#4
# 3
LU
E
%
67 olo
5
66
R
T
Y
&
7
AcGraw Hill LLC. All Rights R
X
8. (16 pts) Provide the stepwise mechanism for the synthesis of the following compound via an enamine
Chapter 6 Solutions
Organic Chemistry
Ch. 6 - Prob. 6.1PCh. 6 - By taking into account electronegativity...Ch. 6 - Use curved arrows to show the movement of...Ch. 6 - Prob. 6.4PCh. 6 - Prob. 6.5PCh. 6 - Prob. 6.6PCh. 6 - aWhich Keq corresponds to a negative value of G,...Ch. 6 - Given each of the following values, is the...Ch. 6 - Given each of the following values, is the...Ch. 6 - The equilibrium constant for the conversion of the...
Ch. 6 - Prob. 6.11PCh. 6 - For a reaction with H=40kJ/mol, decide which of...Ch. 6 - For a reaction with H=20kJ/mol, decide which of...Ch. 6 - Draw an energy diagram for a reaction in which the...Ch. 6 - Prob. 6.15PCh. 6 - Prob. 6.16PCh. 6 - Problem 6.19 Consider the following energy...Ch. 6 - Draw an energy diagram for a two-step reaction,...Ch. 6 - Which value if any corresponds to a faster...Ch. 6 - Prob. 6.20PCh. 6 - Problem 6.23 For each rate equation, what effect...Ch. 6 - Prob. 6.22PCh. 6 - Identify the catalyst in each equation. a....Ch. 6 - Draw the products of homolysis or heterolysis of...Ch. 6 - Explain why the bond dissociation energy for bond...Ch. 6 - Classify each transformation as substitution,...Ch. 6 - Prob. 6.27PCh. 6 - Draw the products of each reaction by following...Ch. 6 - Prob. 6.29PCh. 6 - Prob. 6.30PCh. 6 - Prob. 6.31PCh. 6 - Prob. 6.32PCh. 6 - Prob. 6.33PCh. 6 - Prob. 6.34PCh. 6 - Prob. 6.35PCh. 6 - 6.39. a. Which value corresponds to a negative...Ch. 6 - Prob. 6.37PCh. 6 - At 25 C, the energy difference Go for the...Ch. 6 - For which of the following reaction is S a...Ch. 6 - Prob. 6.40PCh. 6 - Prob. 6.41PCh. 6 - 6.44 Consider the following reaction: .
Use curved...Ch. 6 - Prob. 6.43PCh. 6 - Draw an energy diagram for the Bronsted-Lowry...Ch. 6 - Prob. 6.45PCh. 6 - Prob. 6.46PCh. 6 - Prob. 6.47PCh. 6 - Prob. 6.48PCh. 6 - The conversion of acetyl chloride to methyl...Ch. 6 - Prob. 6.50PCh. 6 - Prob. 6.51PCh. 6 - 6.54 Explain why is more acidic than , even...Ch. 6 - Prob. 6.53PCh. 6 - Prob. 6.54PCh. 6 - Prob. 6.55PCh. 6 - Although Keq of equation 1 in problem 6.57 does...Ch. 6 - Prob. 6.57P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the titration curve of (i) weak acid vs. strong base; (ii) weak acid vs. weakbase; (iii) diprotic acid with strong base (iii) triprotic acid with strong base.arrow_forwardComplete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. my ㄖˋ + 1. Na O Me Click and drag to start drawing a structure. 2. H +arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe H+ + 1 2 H H work up You can draw 1 and 2 in any arrangement you like. Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Click and drag to start drawing a structure. X $ dmarrow_forward
- Predict the major products of this organic reaction: 1. NaH (20°C) 2. CH3Br ? Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. G Crarrow_forwardPredict the major products of this organic reaction: 1. LDA (-78°C) ? 2. Br Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. . • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. Xarrow_forwardPlease draw the structuresarrow_forward
- Draw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 0 1. Eto 1. Eto- 1 2 2. MeBr 2. EtBr H3O+ A 3 You can draw the three structures in any arrangement you like. Explanation Check Click and drag to start drawing a structure.arrow_forwardDraw the missing intermediate 1 and final product 2 of this synthesis: 1. MeO- H3O+ 1 2 2. PrBr Δ You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardWhat is the differences between: Glyceride and phosphoglyceride Wax and Fat Soap and Fatty acid HDL and LDL cholesterol Phospho lipids and sphingosine What are the types of lipids? What are the main lipid components of membrane structures? How could lipids play important rules as signaling molecules and building units? The structure variety of lipids makes them to play significant rules in our body, conclude breifly on this statement.arrow_forward
- What is the differences between DNA and RNA for the following: - structure - function - type What is the meaning of: - replication - transcription - translation show the base pair connection(hydrogen bond) in DNA and RNAarrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY